Summary
Nitrobenzene is a widely used organic compound with a diverse range of applications, from the production of dyes and pharmaceuticals to the synthesis of other chemical compounds. Understanding the density of nitrobenzene is crucial for various scientific and industrial processes. This comprehensive guide delves into the intricacies of nitrobenzene density, providing physics students with a detailed and technical exploration of this important property.
Understanding Nitrobenzene Density
Nitrobenzene, with the chemical formula C₆H₅NO₂, is a pale yellow, oily liquid with a distinctive almond-like odor. Its density, a fundamental physical property, is a measure of the mass per unit volume of the substance. The density of nitrobenzene is an essential parameter in various applications, such as chemical reactions, separation processes, and material handling.
Theoretical Aspects of Nitrobenzene Density
The density of nitrobenzene can be calculated using the following formula:
ρ = m / V
Where:
– ρ is the density of nitrobenzene (in g/cm³)
– m is the mass of the nitrobenzene sample (in grams)
– V is the volume of the nitrobenzene sample (in cubic centimeters)
The density of nitrobenzene is influenced by several factors, including temperature, pressure, and the presence of impurities or other substances. As the temperature of nitrobenzene increases, its density typically decreases due to the expansion of the liquid. Conversely, an increase in pressure can lead to a slight increase in the density of nitrobenzene.
Experimental Determination of Nitrobenzene Density
The density of nitrobenzene can be experimentally determined using various techniques, such as the pycnometer method, the buoyancy method, or the use of a digital density meter. These methods involve precisely measuring the mass and volume of a known quantity of nitrobenzene, allowing for the calculation of its density.
Pycnometer Method
The pycnometer method is a widely used technique for determining the density of liquids, including nitrobenzene. It involves the use of a calibrated glass vessel, known as a pycnometer, with a precisely known volume. The steps involved in the pycnometer method are as follows:
- Weigh the empty, clean, and dry pycnometer.
- Fill the pycnometer with the nitrobenzene sample, ensuring that there are no air bubbles present.
- Weigh the pycnometer filled with the nitrobenzene sample.
- Calculate the density of nitrobenzene using the formula:
ρ = (m₂ - m₁) / V
Where:
– ρ is the density of nitrobenzene (in g/cm³)
– m₁ is the mass of the empty pycnometer (in grams)
– m₂ is the mass of the pycnometer filled with nitrobenzene (in grams)
– V is the known volume of the pycnometer (in cubic centimeters)
Buoyancy Method
The buoyancy method is another technique used to determine the density of liquids, including nitrobenzene. This method relies on the principle of Archimedes’ principle, which states that the buoyant force acting on an object immersed in a liquid is equal to the weight of the liquid displaced by the object.
The steps involved in the buoyancy method are as follows:
- Weigh a solid object (e.g., a metal sinker) in air.
- Immerse the object in the nitrobenzene sample and weigh it again.
- Calculate the density of nitrobenzene using the formula:
ρ = (m₁ - m₂) / V
Where:
– ρ is the density of nitrobenzene (in g/cm³)
– m₁ is the mass of the object in air (in grams)
– m₂ is the mass of the object immersed in nitrobenzene (in grams)
– V is the volume of the object (in cubic centimeters)
Digital Density Meter
Modern technology has introduced digital density meters, which provide a convenient and accurate way to determine the density of liquids, including nitrobenzene. These instruments use various techniques, such as the oscillating U-tube method or the vibrating wire method, to measure the density of the sample directly.
The steps involved in using a digital density meter to determine the density of nitrobenzene are as follows:
- Calibrate the density meter according to the manufacturer’s instructions, typically using a reference liquid with a known density.
- Introduce the nitrobenzene sample into the measurement cell of the density meter.
- The density meter will automatically measure and display the density of the nitrobenzene sample.
Factors Affecting Nitrobenzene Density
The density of nitrobenzene can be influenced by several factors, including temperature, pressure, and the presence of impurities or other substances.
Temperature
As mentioned earlier, the density of nitrobenzene is inversely proportional to temperature. As the temperature of nitrobenzene increases, its volume expands, leading to a decrease in density. This relationship can be expressed using the following formula:
ρ₂ = ρ₁ / (1 + α(T₂ - T₁))
Where:
– ρ₁ is the density of nitrobenzene at the initial temperature T₁ (in g/cm³)
– ρ₂ is the density of nitrobenzene at the final temperature T₂ (in g/cm³)
– α is the coefficient of thermal expansion of nitrobenzene (in °C⁻¹)
The coefficient of thermal expansion for nitrobenzene is approximately 0.00096 °C⁻¹.
Pressure
The density of nitrobenzene is also affected by changes in pressure, although the effect is relatively small compared to the influence of temperature. As the pressure on the nitrobenzene sample increases, its density slightly increases due to the compression of the liquid.
The relationship between the density of nitrobenzene and pressure can be expressed using the following formula:
ρ₂ = ρ₁ / (1 - (P₂ - P₁) / K)
Where:
– ρ₁ is the density of nitrobenzene at the initial pressure P₁ (in g/cm³)
– ρ₂ is the density of nitrobenzene at the final pressure P₂ (in g/cm³)
– P₁ and P₂ are the initial and final pressures, respectively (in pascals)
– K is the bulk modulus of nitrobenzene (in pascals)
The bulk modulus of nitrobenzene is approximately 1.2 × 10⁹ Pa.
Impurities and Other Substances
The presence of impurities or other substances in nitrobenzene can also affect its density. The addition of a solute or other compounds to the nitrobenzene sample can either increase or decrease the overall density, depending on the properties of the added substance.
Nitrobenzene Density Data and Examples
The density of nitrobenzene at various temperatures and pressures is provided in the table below:
| Temperature (°C) | Density (g/cm³) |
|---|---|
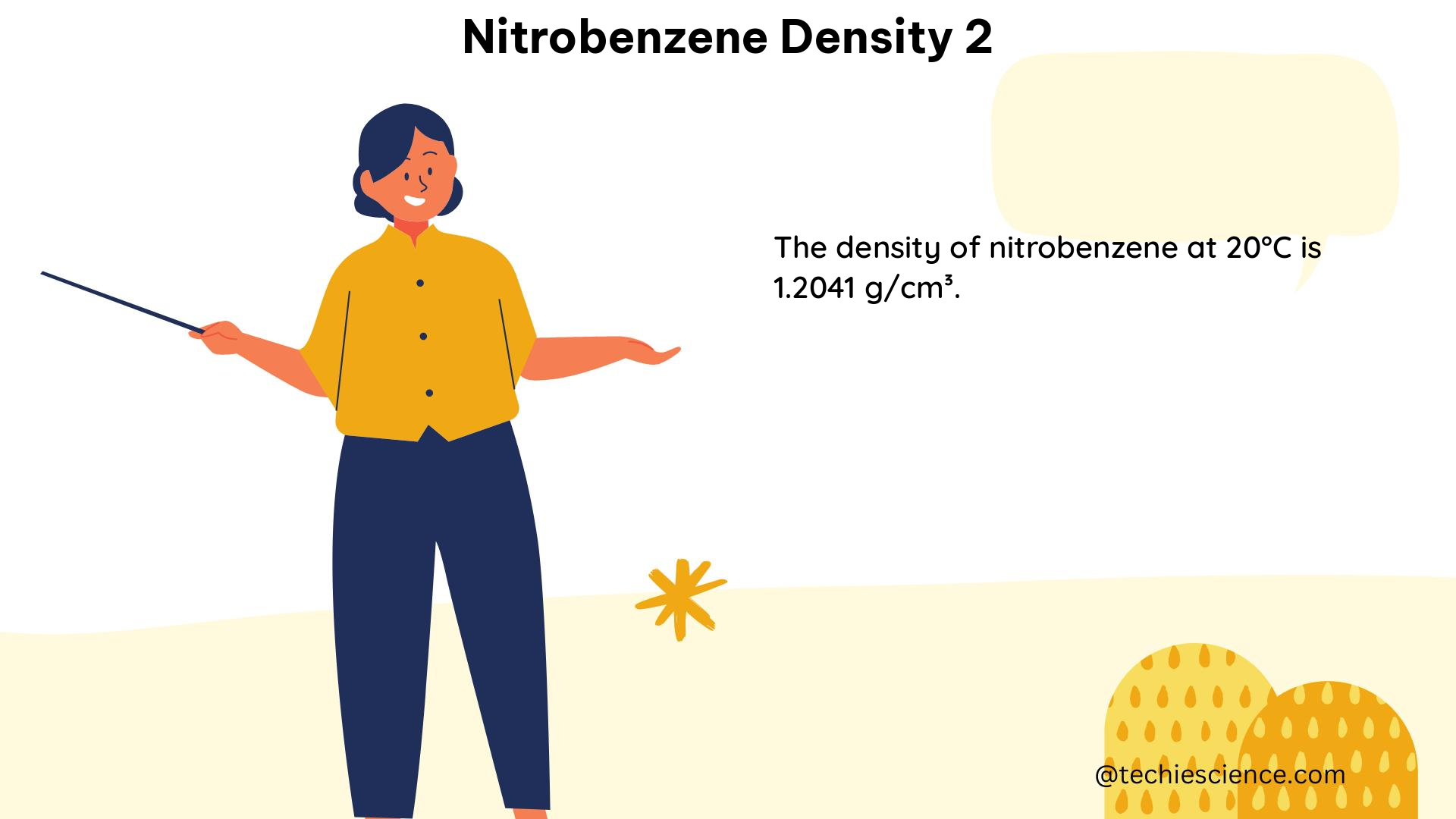
| 0 | 1.2048 |
| 10 | 1.2009 |
| 20 | 1.1970 |
| 30 | 1.1931 |
| 40 | 1.1892 |
| 50 | 1.1853 |
Example 1:
Determine the density of nitrobenzene at 25°C and 1 atm pressure.
Given:
– Temperature, T = 25°C
– Pressure, P = 1 atm = 101.325 kPa
Using the formula for the effect of temperature on density:
ρ₂ = ρ₁ / (1 + α(T₂ – T₁))
Where:
– ρ₁ = 1.1970 g/cm³ (from the table at 20°C)
– α = 0.00096 °C⁻¹
– T₁ = 20°C
– T₂ = 25°C
Substituting the values:
ρ₂ = 1.1970 / (1 + 0.00096(25 – 20))
ρ₂ = 1.1951 g/cm³
Therefore, the density of nitrobenzene at 25°C and 1 atm pressure is 1.1951 g/cm³.
Example 2:
Calculate the density of nitrobenzene at 35°C and 2 atm pressure.
Given:
– Temperature, T = 35°C
– Pressure, P = 2 atm = 202.65 kPa
Using the formula for the effect of temperature on density:
ρ₂ = ρ₁ / (1 + α(T₂ – T₁))
Where:
– ρ₁ = 1.1931 g/cm³ (from the table at 30°C)
– α = 0.00096 °C⁻¹
– T₁ = 30°C
– T₂ = 35°C
Substituting the values:
ρ₂ = 1.1931 / (1 + 0.00096(35 – 30))
ρ₂ = 1.1892 g/cm³
Using the formula for the effect of pressure on density:
ρ₃ = ρ₂ / (1 – (P₂ – P₁) / K)
Where:
– ρ₂ = 1.1892 g/cm³ (from the previous step)
– P₁ = 1 atm = 101.325 kPa
– P₂ = 2 atm = 202.65 kPa
– K = 1.2 × 10⁹ Pa
Substituting the values:
ρ₃ = 1.1892 / (1 – (202.65 – 101.325) / (1.2 × 10⁹))
ρ₃ = 1.1893 g/cm³
Therefore, the density of nitrobenzene at 35°C and 2 atm pressure is 1.1893 g/cm³.
These examples demonstrate the application of the formulas and data provided to calculate the density of nitrobenzene under different temperature and pressure conditions.
Conclusion
In conclusion, the density of nitrobenzene is a crucial physical property that plays a significant role in various scientific and industrial applications. This comprehensive guide has provided physics students with a detailed understanding of the theoretical aspects, experimental determination, and factors affecting the density of nitrobenzene. By mastering the concepts and techniques presented in this guide, students can confidently apply their knowledge to solve real-world problems and contribute to the advancement of scientific research and industrial processes involving nitrobenzene.
References:
- Lide, D. R. (Ed.). (2005). CRC Handbook of Chemistry and Physics (86th ed.). CRC Press.
- Haynes, W. M. (Ed.). (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press.
- Weast, R. C. (Ed.). (1984). CRC Handbook of Chemistry and Physics (64th ed.). CRC Press.
- Riddick, J. A., Bunger, W. B., & Sakano, T. K. (1986). Organic Solvents: Physical Properties and Methods of Purification (4th ed.). Wiley-Interscience.
- Yaws, C. L. (2003). Yaws’ Handbook of Thermodynamic and Physical Properties of Chemical Compounds. Knovel.

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.