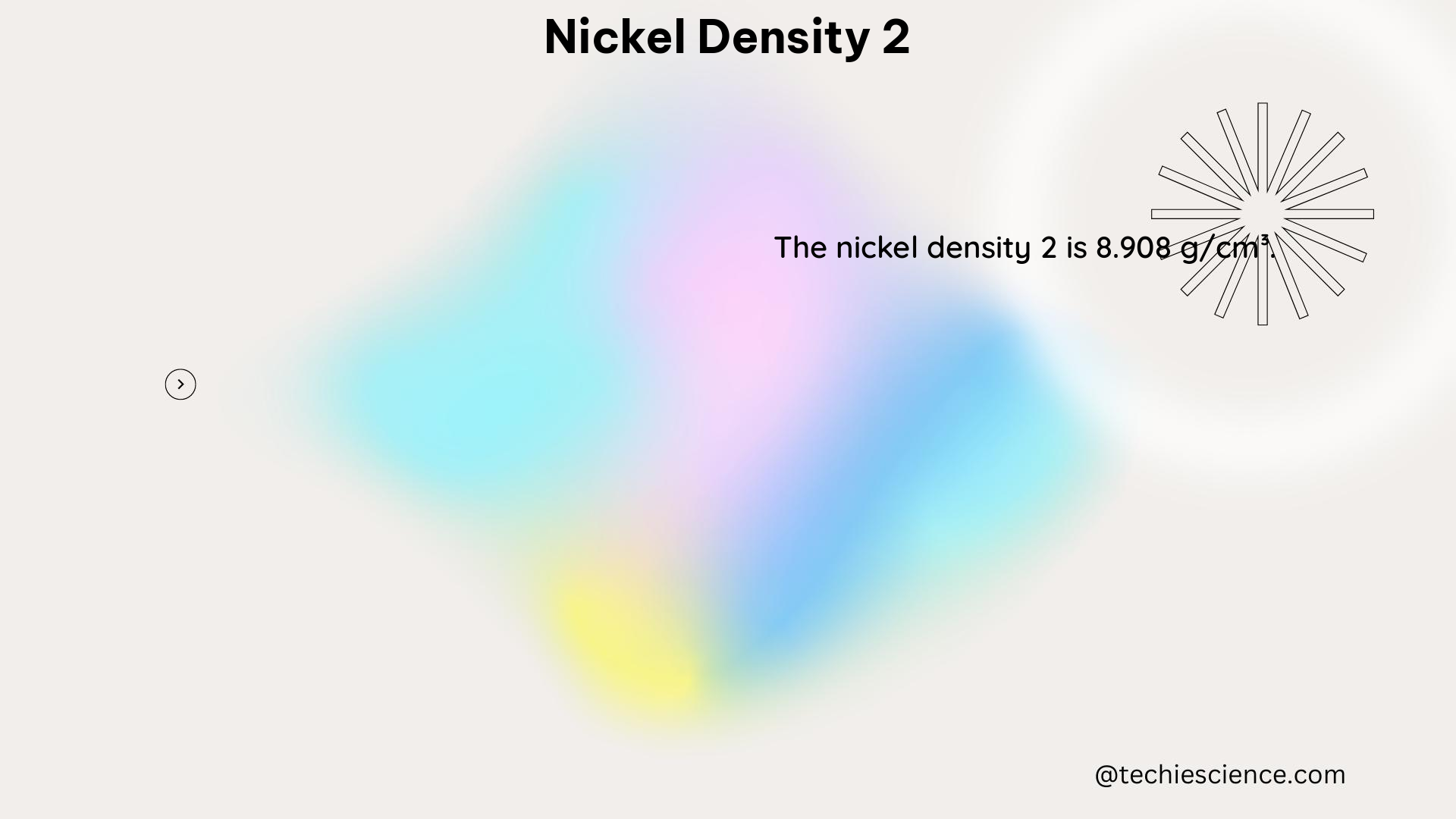
Nickel density 2, also known as nickel-based superalloys, is a class of high-performance materials that have a density of approximately 8.9 g/cm³. These alloys are primarily composed of nickel, chromium, and molybdenum, with smaller amounts of other elements such as cobalt, aluminum, and titanium. The specific density of nickel density 2 can vary slightly depending on the exact composition of the alloy.
Understanding the Composition and Properties of Nickel Density 2
Nickel density 2 is a complex alloy that is designed to withstand extreme temperatures and harsh environments. The key elements that contribute to its unique properties are:
-
Nickel (Ni): Nickel is the primary constituent of nickel density 2, typically making up around 50-70% of the alloy. Nickel provides the alloy with its high strength, corrosion resistance, and ductility.
-
Chromium (Cr): Chromium is added to the alloy to enhance its oxidation resistance and high-temperature strength. Chromium forms a protective oxide layer on the surface of the alloy, which helps to prevent corrosion and degradation at elevated temperatures.
-
Molybdenum (Mo): Molybdenum is added to the alloy to improve its strength, creep resistance, and thermal stability. Molybdenum also helps to stabilize the alloy’s microstructure, which is crucial for maintaining its mechanical properties at high temperatures.
-
Other Elements: Smaller amounts of elements such as cobalt, aluminum, and titanium are often added to nickel density 2 to further enhance its properties. Cobalt improves the alloy’s high-temperature strength, while aluminum and titanium can increase its oxidation resistance and creep resistance.
The combination of these elements in the right proportions gives nickel density 2 its unique set of properties, which include:
-
High Strength: Nickel density 2 has an exceptionally high strength-to-weight ratio, making it an ideal material for applications where weight is a critical factor, such as in aerospace and power generation industries.
-
Excellent Corrosion Resistance: The alloy’s protective oxide layer and chemical composition make it highly resistant to corrosion, even in harsh environments.
-
Good Ductility: Nickel density 2 exhibits good ductility, which allows it to be easily fabricated and shaped into complex components.
- Thermal Stability: The alloy can maintain its mechanical properties and microstructural integrity even at extremely high temperatures, making it suitable for use in high-temperature applications.
Measuring the Density of Nickel Density 2
To measure the density of nickel density 2, researchers and engineers typically use two main methods:
- Gravimetry: This method involves measuring the mass of a sample and its volume, and then calculating the density based on these measurements. The formula for density using the gravimetric method is:
Density = Mass / Volume
The volume of the sample can be determined using various techniques, such as water displacement or dimensional measurements.
- Hydrostatic Weighing: This method involves measuring the weight of a sample in air and then in a liquid with a known density, such as water. The density of the sample can then be calculated using the principles of buoyancy, as described by Archimedes’ principle:
Density = (Weight in air) / (Weight in air - Weight in liquid)
The weight of the sample in the liquid is less than its weight in air due to the buoyant force acting on the sample, which is proportional to the density of the liquid and the volume of the sample.
Both gravimetry and hydrostatic weighing are widely used to determine the density of nickel density 2 and other metallic alloys. The choice of method depends on the available equipment, the size and shape of the sample, and the desired accuracy of the measurement.
Applying the Principles of Physics to Nickel Density 2
When working with nickel density 2 in a physics context, it is essential to understand how its properties relate to the principles of thermodynamics and material science. Some key considerations include:
-
Thermal Expansion: Nickel density 2 has a relatively low coefficient of thermal expansion, which means that it undergoes minimal dimensional changes when exposed to high temperatures. This property is crucial in applications where thermal stability is critical, such as in gas turbines and jet engines.
-
Thermal Conductivity: The thermal conductivity of nickel density 2 is generally lower than that of pure nickel, which helps to minimize heat transfer and maintain the alloy’s structural integrity at high temperatures.
-
Specific Heat Capacity: Nickel density 2 has a relatively high specific heat capacity, meaning it can absorb and store a significant amount of thermal energy without experiencing a large temperature change. This property is beneficial in applications where the material is subjected to rapid temperature fluctuations.
-
Mechanical Properties: The high strength, ductility, and creep resistance of nickel density 2 are directly related to its microstructure and the interactions between the various alloying elements. Understanding the principles of materials science, such as phase transformations and dislocation mechanics, is crucial for predicting and optimizing the mechanical behavior of the alloy.
-
Oxidation and Corrosion: The excellent oxidation and corrosion resistance of nickel density 2 are a result of the formation of a protective oxide layer on the surface of the alloy. This layer acts as a barrier, preventing further degradation of the material. The principles of electrochemistry and thermodynamics can be used to understand the mechanisms behind this behavior.
By applying the principles of physics and materials science to the study of nickel density 2, students and researchers can gain a deeper understanding of the alloy’s unique properties and how they can be leveraged in various applications.
Numerical Examples and Calculations
To further illustrate the properties of nickel density 2, let’s consider some numerical examples and calculations:
- Density Calculation: Suppose a sample of nickel density 2 has a mass of 50 grams and a volume of 5.6 cm³. Using the gravimetric method, the density of the sample can be calculated as:
Density = Mass / Volume
Density = 50 g / 5.6 cm³ = 8.93 g/cm³
This value is consistent with the typical density range of nickel density 2, which is around 8.9 g/cm³.
- Thermal Expansion Coefficient: The coefficient of thermal expansion (CTE) for nickel density 2 is typically in the range of 12-15 × 10^-6 per °C. This means that for every 1°C increase in temperature, the material will expand by 12-15 parts per million of its original length.
For example, if a component made of nickel density 2 has an initial length of 100 mm, and the temperature increases by 100°C, the change in length can be calculated as:
Change in length = Initial length × CTE × Temperature change
Change in length = 100 mm × 13 × 10^-6 per °C × 100°C = 0.13 mm
This small change in length is crucial in maintaining the dimensional stability of nickel density 2 components in high-temperature applications.
- Specific Heat Capacity: The specific heat capacity of nickel density 2 is typically around 0.435 J/g·°C. This means that it takes 0.435 joules of energy to raise the temperature of 1 gram of the alloy by 1°C.
For instance, if a 1 kg (1000 g) block of nickel density 2 is heated from 20°C to 500°C, the amount of energy required can be calculated as:
Energy required = Mass × Specific heat capacity × Temperature change
Energy required = 1000 g × 0.435 J/g·°C × (500°C - 20°C) = 208,800 J
This high specific heat capacity allows nickel density 2 to effectively absorb and store thermal energy, which is beneficial in applications where the material is subjected to rapid temperature changes.
These examples demonstrate how the physical properties of nickel density 2, such as density, thermal expansion, and specific heat capacity, can be quantified and applied in various engineering and scientific contexts.
Conclusion
Nickel density 2, or nickel-based superalloys, is a highly specialized class of materials that possess a unique combination of properties, including high strength, excellent corrosion resistance, and thermal stability. Understanding the composition, structure, and physics-based principles that govern the behavior of these alloys is crucial for students and researchers working in fields such as materials science, aerospace engineering, and power generation.
By mastering the concepts and techniques presented in this comprehensive guide, you will be well-equipped to tackle the challenges and opportunities presented by nickel density 2 in both theoretical and practical applications.
Reference:
- Nickel Institute – Nickel in Alloys
- ASM International – Nickel-Based Superalloys
- NIST – Nickel Properties

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.