Nickel is a versatile and widely used metal, known for its high density, corrosion resistance, and magnetic properties. Understanding the precise density of nickel is crucial in various industries, from mining and processing to manufacturing and engineering. This comprehensive guide delves into the intricacies of nickel density, providing a wealth of technical details and practical applications for physics students and professionals alike.
The Density of Nickel: A Fundamental Property
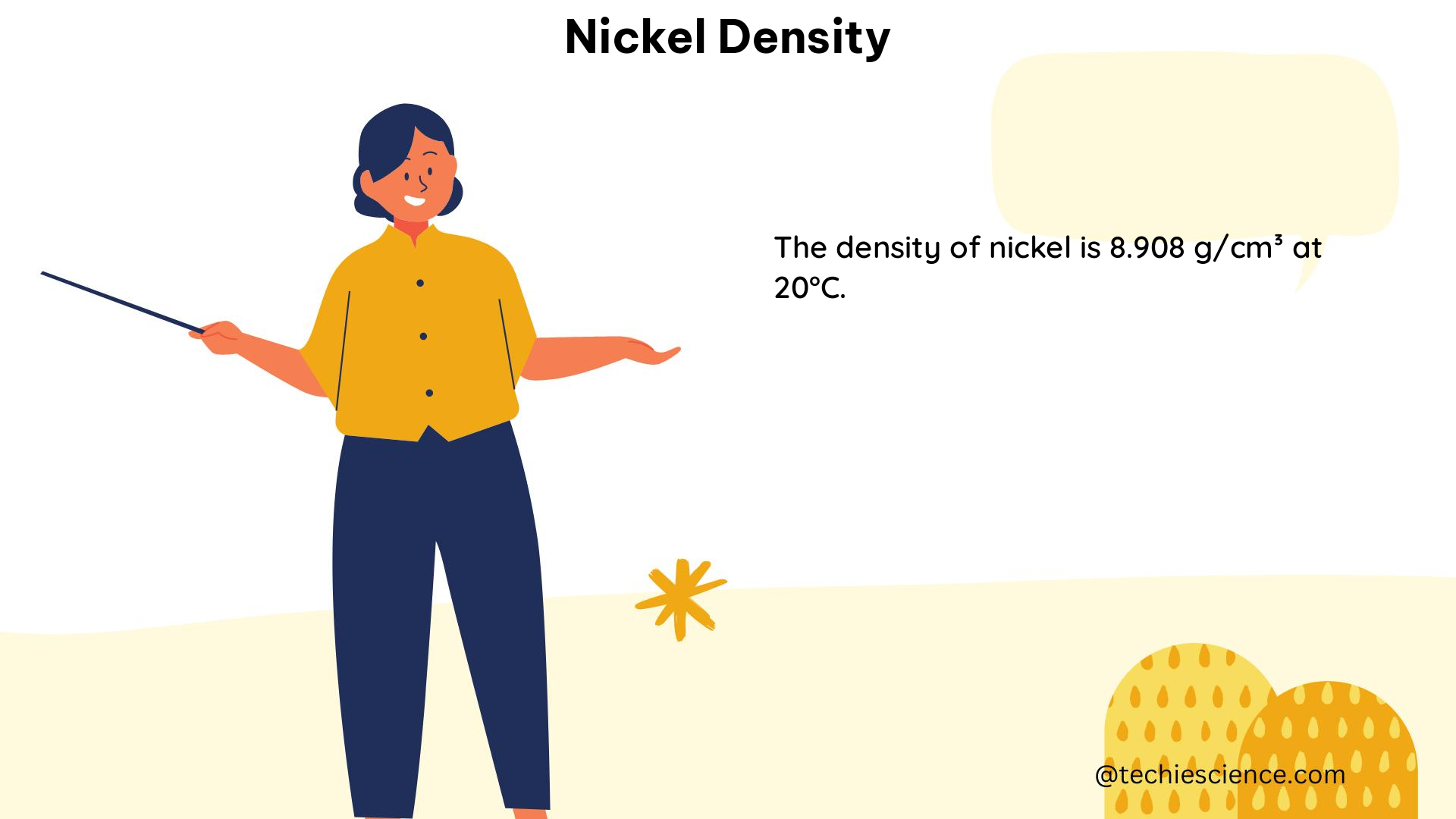
Nickel has a density of approximately 8.9 g/cm³, which means that a cubic centimeter of pure nickel has a mass of approximately 8.9 grams. This high density is a result of the metal’s atomic structure and the strong intermolecular forces that hold its atoms together.
The density of nickel can be calculated using the formula:
ρ = m / V
Where:
– ρ is the density of the material (in this case, nickel)
– m is the mass of the material
– V is the volume of the material
For a sample of pure nickel with a mass of 10 grams and a volume of 1.12 cm³, the density can be calculated as:
ρ = 10 g / 1.12 cm³ = 8.93 g/cm³
This value is consistent with the commonly reported density of nickel, which can vary slightly depending on the purity and temperature of the sample.
Factors Affecting Nickel Density
The density of nickel can be influenced by several factors, including:
-
Purity: The presence of impurities or alloying elements in the nickel can affect its density. Higher purity nickel will generally have a higher density.
-
Temperature: The density of nickel, like most materials, decreases as the temperature increases. This is due to the thermal expansion of the metal, which causes the atoms to move further apart, reducing the overall density.
-
Pressure: Increased pressure can cause the atoms in nickel to be packed more tightly, resulting in a higher density. This effect is particularly noticeable at extremely high pressures, such as those found in the Earth’s core.
-
Crystalline Structure: The specific crystalline structure of nickel can also influence its density. Nickel typically has a face-centered cubic (FCC) crystal structure, but other structures, such as the hexagonal close-packed (HCP) structure, can occur under certain conditions and may have slightly different densities.
To illustrate the effect of these factors, consider the following examples:
Example 1: Nickel Alloy Density
A nickel-chromium alloy with a composition of 80% nickel and 20% chromium has a density of approximately 8.4 g/cm³, which is slightly lower than pure nickel due to the presence of the chromium atoms.
Example 2: Temperature and Nickel Density
The density of nickel at 0°C is 8.902 g/cm³, while at 1,455°C (the melting point of nickel), the density decreases to 7.81 g/cm³, a reduction of approximately 12%.
Example 3: Pressure and Nickel Density
Under extremely high pressures, such as those found in the Earth’s core (approximately 360 GPa), the density of nickel can increase to around 12.5 g/cm³, a significant increase compared to its standard density at atmospheric pressure.
Measuring Nickel Density
Accurately measuring the density of nickel is crucial in various industries, from mining and processing to manufacturing and quality control. Several techniques are commonly used to determine the density of nickel, including:
-
Hydrostatic Weighing: This method involves weighing a sample of nickel in air and then weighing it while submerged in a liquid, typically water. The difference in weight, combined with the known density of the liquid, can be used to calculate the density of the nickel sample.
-
Pycnometry: This technique uses a calibrated glass vessel, called a pycnometer, to measure the volume of a known mass of nickel. The density is then calculated by dividing the mass by the measured volume.
-
Helium Pycnometry: This method uses helium gas to measure the volume of a nickel sample, which is then used to calculate the density. Helium is chosen because it can penetrate even the smallest pores in the material, providing a more accurate volume measurement.
-
Gamma-Ray Attenuation: This non-destructive technique uses a gamma-ray source and a detector to measure the attenuation of the gamma rays as they pass through a nickel sample. The degree of attenuation is directly related to the density of the material, allowing for the calculation of the nickel’s density.
-
Ultrasonic Density Measurement: This method uses high-frequency sound waves to determine the density of nickel. The speed of sound through the material is related to its density, allowing for the calculation of the nickel’s density.
Each of these techniques has its own advantages and limitations, and the choice of method will depend on factors such as the sample size, the required accuracy, and the available equipment.
Nickel Density Applications
The high density of nickel makes it a valuable material in a wide range of applications, including:
-
Mining and Processing: Accurate density measurements are crucial in the mining and processing of nickel ores, as they help ensure that order volumes are met and prevent costly damage to equipment.
-
Aerospace and Defense: Nickel’s high density and strength-to-weight ratio make it a popular choice for components in aircraft, spacecraft, and military equipment.
-
Electroplating and Coatings: Nickel is commonly used in electroplating and coating processes to provide corrosion resistance, hardness, and a decorative finish to various products.
-
Catalysts: The high density of nickel active sites on catalysts can influence their performance and deactivation, making it an important parameter in catalyst design and optimization.
-
Alloy Development: The density of nickel is a key factor in the development of nickel-based alloys, as it affects the overall weight and mechanical properties of the final product.
-
Energy Storage: Nickel-based batteries, such as nickel-cadmium and nickel-metal hydride batteries, rely on the high density of nickel to store and release energy efficiently.
-
Jewelry and Decorative Applications: The high density and luster of nickel make it a popular choice for jewelry and other decorative items.
By understanding the intricacies of nickel density, professionals in these industries can optimize their processes, develop better products, and ensure compliance with industry standards and regulations.
Conclusion
Nickel’s high density of approximately 8.9 g/cm³ is a fundamental property that has far-reaching implications across various industries. This comprehensive guide has explored the factors that influence nickel density, the techniques used to measure it, and the diverse applications that rely on this critical parameter.
Whether you’re a physics student, a mining engineer, or a materials scientist, this guide has provided you with a wealth of technical details and practical insights to deepen your understanding of nickel density and its importance in the real world. By mastering the concepts presented here, you’ll be better equipped to tackle the challenges and opportunities that arise in the dynamic world of nickel-based materials and technologies.
References
- Quantification of nickel, cobalt, and manganese concentration using ultraviolet-visible spectroscopy, published in RSC Advances, 2021. https://pubs.rsc.org/en/content/articlelanding/2021/ra/d1ra03962h
- The importance of accurate density measurement in nickel mining, published in Mining Technology, 2019. https://www.mining-technology.com/sponsored/the-importance-of-accurate-density-measurement-in-nickel-mining/
- Effect of Nickel Active Site Density on the Deactivation of Ni-Beta, published in ACS Engineering Au, 2021. https://pubs.acs.org/doi/10.1021/acsengineeringau.1c00014
- Quantification of copper, nickel and other elements in copper-nickel ore powders using laser-induced breakdown spectroscopy, published in Spectrochimica Acta Part B: Atomic Spectroscopy, 2019. https://www.sciencedirect.com/science/article/abs/pii/S0584854719301524

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.