Neodymium is a rare-earth metal. Let us discuss the details of Neodymium in this article.
Neodymium is a silvery white metal. It reacts with oxygen to form neodymium(lll) oxide. It reacts with cold and hot water to form neodymium(lll) hydroxide. It reacts with all the stable halogens.
Let us find out the details about the period, Lanthanide series, atomic number, atomic weight, of Neodymium in this article.
Neodymium symbol
The symbol of Neodymium is Nd.
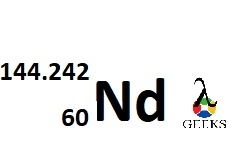
Neodymium group in the periodic table
Neodymium occupies the Lanthanide series. It occupies the fourth position in the Lanthanide series.
Neodymium period in the periodic table
Neodymium belongs to period 6.
Neodymium block in the periodic table
Neodymium exists in the f block of the periodic table.
Neodymium atomic number
The atomic number of Neodymium is 60.
Neodymium atomic weight
The atomic weight of Neodymium is 144.242 g/mol
Neodymium Electronegativity according to Pauling
The electronegativity of Neodymium on Pauling scale is 1.14.
Neodymium atomic density
The atomic density of Neodymium is 7.01 g/cm3.
Neodymium melting point
The melting point of Neodymium is 1297 K (1,024 οC).
Neodymium boiling point
The boiling point of Neodymium is 3347 K (3,074 οC).
Neodymium Van der waals radius
The Van der waals radius of Neodymium is (empirical) 181 pm.
Neodymium ionic/covalent radius
The covalent radius of Neodymium is 201±6 pm.
Neodymium isotopes
Neodymium has 5 stable isotopes. Let us explore the isotopes of Neodymium below.
The main isotopes of Neodymium are as follows-
| Isotopes | Abundance | Half-life |
|---|---|---|
| 142Nd | 27.2% | stable |
| 143Nd | 12.2% | stable |
| 144Nd | 23.8% | 2.29×1015 years |
| 145Nd | 8.3% | stable |
| 146Nd | 17.2% | stable |
| 148Nd | 5.8% | stable |
| 150Nd | 5.6% | 6.7×1018 years |
Neodymium electronic shell
The electrons are arranged in the different electronic shells in the element. Let us find out for Neodymium.
Neodymium consists of 6 electronic shells. Those are 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f4 5s2 5p6 6s2 .
Neodymium energy of first ionization
The first ionization energy of Neodymium is 533.1 kJ/mol.
Neodymium energy of second ionization
The second ionization energy of Neodymium is 1040 kJ/mol.
Neodymium energy of third ionization
The third ionization energy of Neodymium is 2130 kJ/mol.
Neodymium oxidation states
The oxidation states of Neodymium is 0, +2, +3, +4.
Neodymium electronic configurations
The Neodymium electronic configuration is [Xe] 4f4 6s2.
Neodymium CAS number
The CAS number Neodymium is 7440-00-8.
Neodymium ChemSpider ID
The ChemSpider ID of Neodymium is 22376.
Neodymium allotropic forms
The Neodymium exists in two allotropic forms.
- α-phase is double close-packed hexagonal at room temperature.
- β-phase is body-centred cubic at 883 οC.
Neodymium chemical classification
Neodymium occupies the f block (lanthanide series) of the periodic table.
Neodymium state at room temperature
Neodymium exists in the solid state at the room temperature.
Is Neodymium paramagnetic?
Magnetic properties of a substance depend on the presence or absence of the unpaired electrons. Let us discuss.
Neodymium is paramagnetic at room temperature but turns antiferromagnetic below 20 K temperature because it contains 4 unpaired electrons.
Conclusion
Neodymium is a rare-earth element. It occupies the fourth place in the lanthanide series. It belongs to period 6.

Hi…I am Sonali Jham. I have done my Post-Graduation in Chemistry and also completed B. Ed. I am a Teacher and a Dietician by profession.
My hobby is reading and painting.
Let’s connect through LinkedIn-