NaOH, or Sodium Hydroxide, is a highly reactive alkali metal hydroxide with a wide range of applications in various industries, including chemistry, pharmaceuticals, and manufacturing. The density of NaOH is a crucial parameter in its handling and usage, as it affects the volume required for specific concentrations and applications. This comprehensive guide will provide you with detailed technical specifications, formulas, examples, and numerical problems to help you understand and calculate the density of NaOH.
Table of Contents
- NaOH Density Preparation
- Dilution Formula
- NaOH Density Calculation
- Physics Examples
- Numerical Problems
- NaOH Density-Concentration Table
- References
NaOH Density Preparation
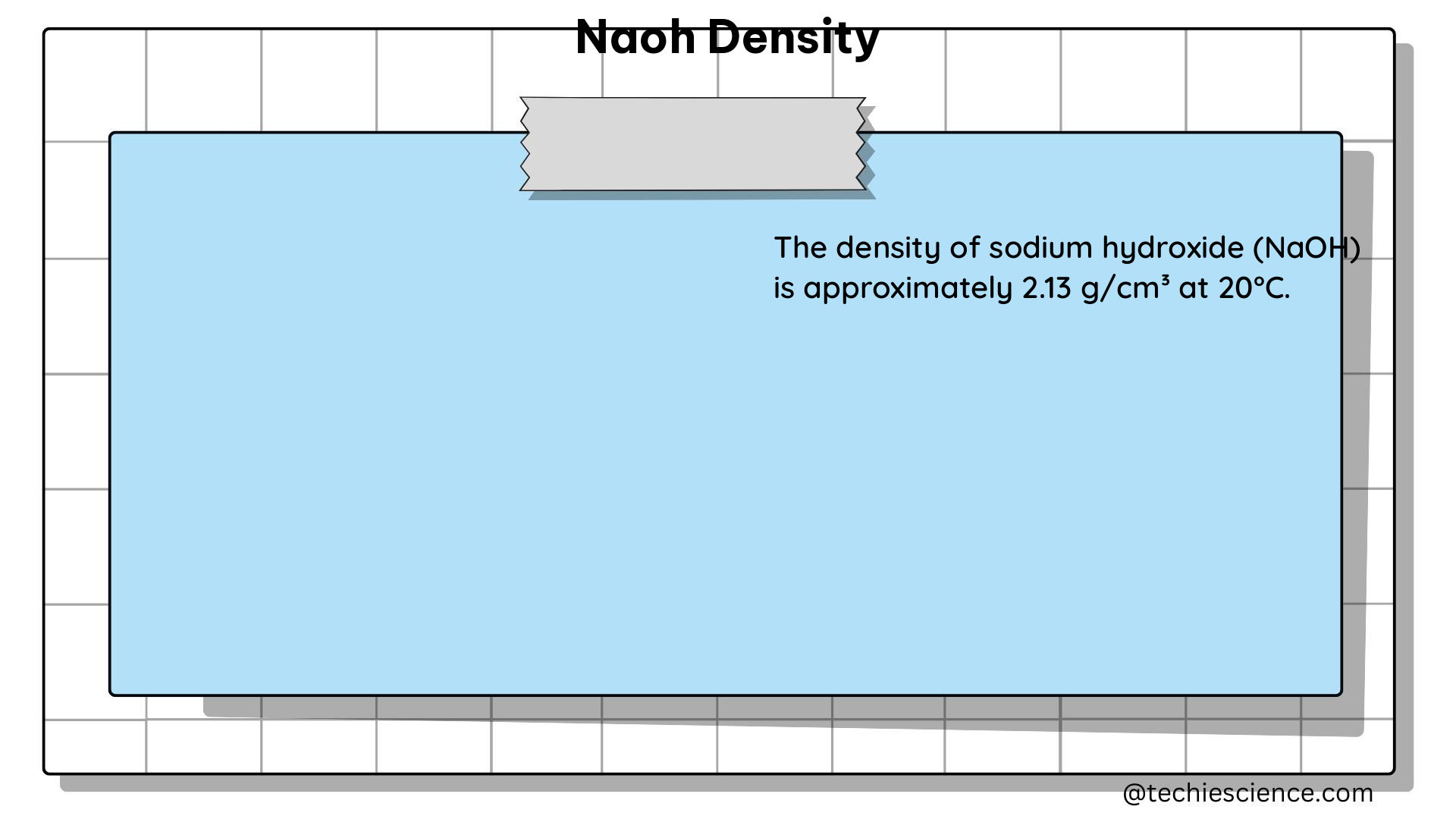
The table below provides essential data for preparing a 0.1 M NaOH solution from a 50% w/w NaOH stock solution. The density of the 50% w/w NaOH solution is 1.53 g/mL, and its molar mass is 39.998 g/mol.
Table 1. Preparation of 0.1 M NaOH from 50% w/w NaOH
| Parameter | Value |
|---|---|
| Density of 50% w/w stock solution | 1.53 g/mL |
| Molar mass NaOH | 39.998 g/mol |
| Mass of NaOH in 50% w/w stock solution (mg) | 153000 |
| Total mass of 50% w/w stock solution | 100000 mg |
| Volume of 50% w/w stock solution needed (V 1) | 65.92 mL |
To prepare 500 mL of a 0.1 M NaOH solution, 65.92 mL of the 50% w/w NaOH solution is required, which is calculated using the dilution formula.
Dilution Formula
The dilution formula is used to determine the volume of the stock solution needed to prepare a solution with a specific concentration and volume. The formula is:
M 1 V 1 = M 2 V 2
Where:
– M 1 = Molarity of the stock solution (50% w/w NaOH)
– V 1 = Volume of the stock solution needed
– M 2 = Molarity of the final solution (0.1 M NaOH)
– V 2 = Volume of the final solution (500 mL)
NaOH Density Calculation
The density of a substance is defined as its mass per unit volume. In the case of NaOH, the density varies depending on its concentration and temperature. The following formula is used to calculate the density of NaOH:
Density (ρ) = Mass (m) / Volume (V)
Where:
– ρ = Density
– m = Mass of NaOH
– V = Volume of NaOH solution
For example, to calculate the density of a 1 M NaOH solution at 25°C, the mass and volume of NaOH need to be determined. Assuming a 1 L solution, the mass of NaOH would be 39.998 g/mol x 1000 mL / 1000 = 39.998 g. The volume of the solution is 1 L or 1000 mL. Therefore, the density would be 39.998 g / 1000 mL = 0.039998 g/mL or 39.998 kg/m³.
Physics Examples
Example 1: Calculate the volume of a 0.1 M NaOH solution required to neutralize 25 mL of a 0.2 M HCl solution.
Solution:
Using the principle of equivalence, the number of moles of H+ ions in the HCl solution is equal to the number of moles of OH- ions in the NaOH solution. Therefore, the volume of the NaOH solution required is:
V 1 (NaOH) = V 2 (HCl) x M 2 (HCl) / M 1 (NaOH)
V 1 = 25 mL x 0.2 M / 0.1 M = 50 mL
Example 2: Calculate the density of a 5 M NaOH solution at 50°C.
Solution:
Assuming a 1 L solution, the mass of NaOH would be 39.998 g/mol x 5 x 1000 mL / 1000 = 199.99 g. The volume of the solution is 1 L or 1000 mL. Therefore, the density would be 199.99 g / 1000 mL = 0.19999 g/mL or 199.99 kg/m³.
Numerical Problems
- Calculate the volume of a 0.5 M NaOH solution required to neutralize 50 mL of a 0.1 M HCl solution.
- Determine the density of a 3 M NaOH solution at 40°C.
- If you have 100 mL of a 2 M NaOH solution, how many grams of NaOH are present in the solution?
- What volume of a 1 M NaOH solution is needed to neutralize 75 mL of a 0.4 M H2SO4 solution?
- Calculate the mass of NaOH required to prepare 250 mL of a 0.8 M NaOH solution.
NaOH Density-Concentration Table
For a more comprehensive understanding of NaOH density, you can refer to the following table that provides the density of NaOH solutions at various concentrations and temperatures:
NaOH Density-Concentration Table
References
- Smaa Koraym at Johns Hopkins University, MD, USA. (n.d.). Lab 38: Acid and Base Concentrations — Procedure. JoVE. https://www.jove.com/science-education/11151/print/procedure
- Chad Kinney, University of Colorado Pueblo. (2020-10-12). Buret Calibration and Stardardization of NaOH Solution. Chem. LibreTexts. https://chem.libretexts.org/Ancillary_Materials/Worksheets/Worksheets:_Analytical_Chemistry_II/Buret_Calibration_and_Stardardization_of_NaOH_Solution
- HandyMath.com. (n.d.). The Complete Sodium Hydroxide Density-Concentration Table. https://www.handymath.com/cgi-bin/naohtble3.cgi?submit=Entry

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.