Summary
NaCl density 2, also known as the density of a 2% sodium chloride solution, is a crucial parameter in various scientific and industrial applications. This comprehensive guide will delve into the intricacies of calculating, measuring, and understanding the density of a 2% NaCl solution, providing physics students with a detailed and practical resource.
Understanding NaCl Density 2
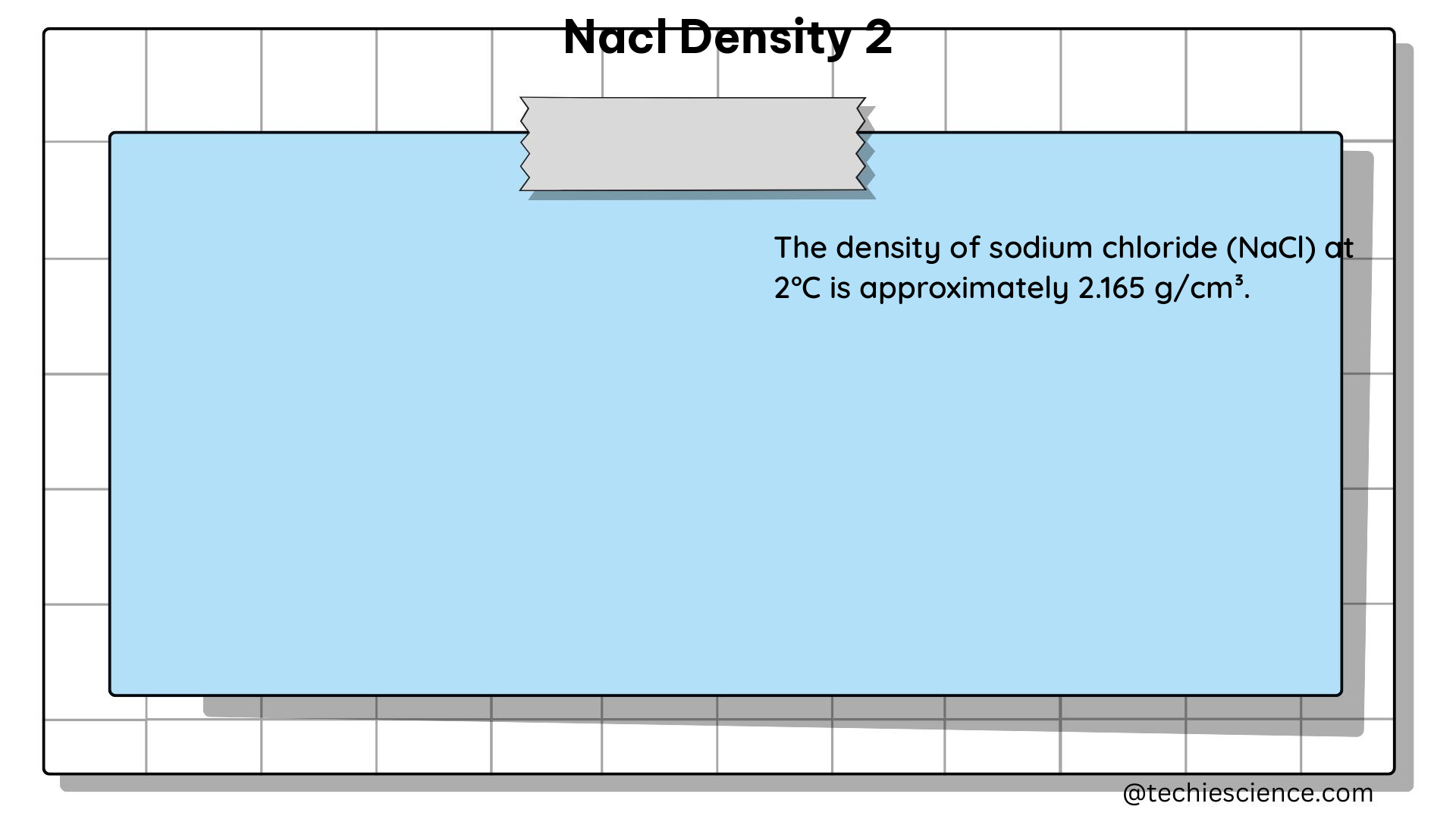
The density of a 2% sodium chloride solution, or NaCl density 2, is the mass per unit volume of the solution. This value is typically expressed in grams per milliliter (g/mL) or kilograms per cubic meter (kg/m³). The density of a solution is influenced by various factors, including the concentration of the solute (in this case, NaCl), temperature, and pressure.
Calculating NaCl Density 2
The formula for calculating the density of a 2% NaCl solution is:
Density = mass / volume
Where:
– Density is the mass per unit volume of the solution, typically expressed in g/mL or kg/m³.
– Mass is the total mass of the NaCl solution, typically measured in grams (g).
– Volume is the total volume of the NaCl solution, typically measured in milliliters (mL) or cubic meters (m³).
To calculate the density of a 2% NaCl solution, you need to know the mass and volume of the solution. This can be determined through experimental measurements or by using the known concentration of the solution.
Experimental Measurements
One way to determine the density of a 2% NaCl solution is through experimental measurements. This involves the following steps:
- Prepare a 2% NaCl solution by dissolving a known mass of NaCl in a known volume of water.
- Measure the mass of the solution using a balance or scale.
- Measure the volume of the solution using a graduated cylinder or volumetric flask.
- Calculate the density using the formula: Density = mass / volume.
The studies mentioned in the original answer provide examples of this experimental approach, where the density of a 2% NaCl solution was found to be approximately 1.01 g/mL and 1.009 g/mL, respectively.
Theoretical Calculations
Alternatively, you can calculate the density of a 2% NaCl solution theoretically, using the known concentration of the solution and the densities of the individual components (water and NaCl).
The density of a solution can be calculated using the following formula:
Density = (mass of solute + mass of solvent) / (volume of solute + volume of solvent)
Where:
– Mass of solute is the mass of NaCl in the solution.
– Mass of solvent is the mass of water in the solution.
– Volume of solute is the volume of NaCl in the solution.
– Volume of solvent is the volume of water in the solution.
By substituting the known values for a 2% NaCl solution, you can calculate the theoretical density of the solution.
Factors Affecting NaCl Density 2
As mentioned in the original answer, the density of a 2% NaCl solution can be affected by various factors, including temperature and pressure.
Temperature
The density of a solution is inversely proportional to its temperature. As the temperature of the solution increases, the density decreases, and vice versa. This is due to the thermal expansion of the solution, which causes the volume to increase more than the mass.
The relationship between temperature and density can be expressed using the following formula:
Density = Density₀ / (1 + α(T – T₀))
Where:
– Density₀ is the density of the solution at the reference temperature T₀.
– α is the thermal expansion coefficient of the solution.
– T is the temperature of the solution.
For a 2% NaCl solution, the thermal expansion coefficient (α) is approximately 0.0002 per degree Celsius (°C).
Pressure
The density of a solution is also affected by pressure. As the pressure on the solution increases, the density increases, and vice versa. This is due to the compressibility of the solution, which causes the volume to decrease more than the mass.
The relationship between pressure and density can be expressed using the following formula:
Density = Density₀ / (1 – β(P – P₀))
Where:
– Density₀ is the density of the solution at the reference pressure P₀.
– β is the compressibility coefficient of the solution.
– P is the pressure of the solution.
For a 2% NaCl solution, the compressibility coefficient (β) is approximately 4.4 × 10⁻⁶ per kilopascal (kPa).
NaCl Density 2 Applications
The density of a 2% NaCl solution has various applications in different fields, including:
-
Chemical Engineering: NaCl density 2 is used in the design and optimization of chemical processes, such as desalination, water treatment, and industrial brine solutions.
-
Oceanography: The density of seawater, which is primarily composed of a 3.5% NaCl solution, is an important parameter in the study of ocean currents, salinity, and other oceanographic phenomena.
-
Biomedical Applications: NaCl density 2 is relevant in the preparation of physiological saline solutions, which are used in various medical procedures and treatments.
-
Food and Beverage Industry: The density of NaCl solutions is crucial in the production and quality control of food and beverage products, such as brines, pickling solutions, and fermentation processes.
-
Environmental Monitoring: The density of NaCl solutions is used in the analysis and monitoring of soil and water samples, particularly in the context of environmental pollution and remediation.
NaCl Density 2 Numerical Examples
To illustrate the practical application of NaCl density 2 calculations, let’s consider the following examples:
Example 1: Calculating the Density of a 2% NaCl Solution
Given:
– Mass of NaCl: 20 g
– Volume of solution: 1000 mL
Calculate the density of the 2% NaCl solution.
Solution:
Density = mass / volume
Density = (20 g + 980 g) / 1000 mL
Density = 1000 g / 1000 mL
Density = 1.00 g/mL
Therefore, the density of the 2% NaCl solution is 1.00 g/mL.
Example 2: Determining the Concentration of NaCl in a Solution
Given:
– Density of the solution: 1.02 g/mL
– Volume of the solution: 500 mL
Calculate the mass of NaCl in the solution and the concentration of NaCl.
Solution:
Mass of the solution = Density × Volume
Mass of the solution = 1.02 g/mL × 500 mL = 510 g
Assuming the solution is a 2% NaCl solution, the mass of NaCl can be calculated as:
Mass of NaCl = 2% of the total mass of the solution
Mass of NaCl = 0.02 × 510 g = 10.2 g
The concentration of NaCl in the solution is:
Concentration of NaCl = (Mass of NaCl / Total mass of solution) × 100%
Concentration of NaCl = (10.2 g / 510 g) × 100% = 2%
Therefore, the mass of NaCl in the solution is 10.2 g, and the concentration of NaCl in the solution is 2%.
Conclusion
NaCl density 2, the density of a 2% sodium chloride solution, is a crucial parameter in various scientific and industrial applications. This comprehensive guide has provided physics students with a detailed understanding of the calculation, measurement, and factors affecting NaCl density 2. By mastering the concepts and techniques presented here, students can confidently apply their knowledge to real-world problems and further their understanding of this important topic in physics.
References
- Strickland, H. (2018). Determination of the Density of a Sodium Chloride Solution Lab Report. Retrieved from https://www.studocu.com/en-us/document/university-of-alabama-at-birmingham/quantitative-chemistry/determination-of-the-density-of-a-sodium-chloride-solution-lab-report/1831927
- CliffsNotes. (n.d.). Percent NaCl Concentration and Density Lab Report 2. Retrieved from https://www.cliffsnotes.com/study-notes/3347545
- Scribd. (n.d.). Density of Aqueous Sodium Chloride Solutions: Experiment 3. Retrieved from https://www.scribd.com/document/401494226/3-Density-Aqueous-Solutions
- Lide, D. R. (2004). CRC Handbook of Chemistry and Physics (84th ed.). CRC Press.
- Haynes, W. M. (2014). CRC Handbook of Chemistry and Physics (95th ed.). CRC Press.

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.