Methanol, a versatile chemical compound, has a well-defined physical property known as its density. This parameter is crucial in various industrial applications and scientific investigations. The density of methanol is approximately 0.791 g/mL at a temperature of 20°C and a pressure of 1 atmosphere, making it slightly lower than the density of water.
Understanding Methanol Density
Methanol density is a crucial parameter that governs the behavior and performance of this chemical in numerous applications. It is essential to have a comprehensive understanding of the factors that influence methanol density, as well as the methods used to measure and quantify this property.
Factors Affecting Methanol Density
-
Temperature: The density of methanol is inversely proportional to temperature. As the temperature increases, the volume of the liquid expands, leading to a decrease in density.
-
Methanol density at 0°C: 0.8107 g/mL
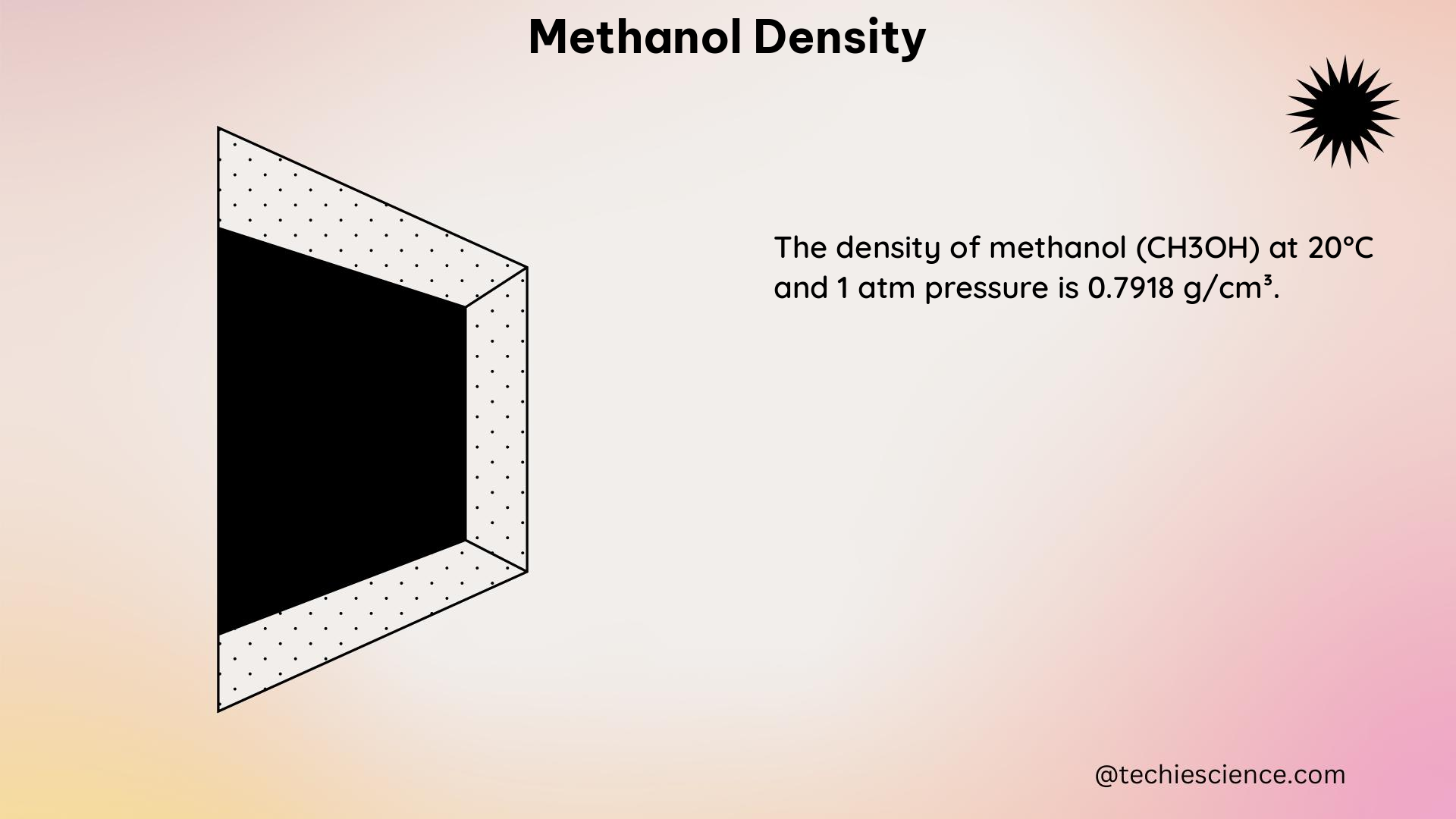
- Methanol density at 20°C: 0.7918 g/mL
-
Methanol density at 40°C: 0.7729 g/mL
-
Pressure: The effect of pressure on methanol density is generally less significant compared to the effect of temperature. However, it is still an important factor to consider in certain applications.
-
Methanol density at 1 atm: 0.7918 g/mL
- Methanol density at 10 atm: 0.7935 g/mL
-
Methanol density at 20 atm: 0.7952 g/mL
-
Concentration: The density of methanol solutions can vary depending on the concentration of methanol in the solution. Higher methanol concentrations typically result in higher solution densities.
-
10% methanol solution density: 0.9818 g/mL
- 50% methanol solution density: 0.9109 g/mL
- 90% methanol solution density: 0.8400 g/mL
Measuring Methanol Density
Accurate measurement of methanol density is crucial in various applications, such as process control, quality assurance, and research. Several methods are available for determining the density of methanol, including:
-
Pycnometry: This method involves the use of a calibrated glass vessel, known as a pycnometer, to measure the mass of a known volume of methanol at a specific temperature.
-
Hydrometry: Hydrometers, which are calibrated floating devices, can be used to measure the density of methanol by determining the depth to which the hydrometer sinks in the liquid.
-
Oscillating U-tube: This technique utilizes an oscillating U-shaped glass tube filled with methanol. The period of oscillation is related to the density of the liquid, allowing for precise density measurements.
-
Density meters: Electronic density meters, such as those based on the principle of vibrating-tube or electromagnetic wave propagation, can provide rapid and accurate measurements of methanol density.
Methanol Density Calculations and Applications
Knowing the density of methanol is essential for various calculations and applications, including:
-
Concentration Determination: The density of methanol solutions can be used to determine the concentration of methanol in a sample, based on a calibration curve relating density to concentration.
-
Volume Calculations: The density of methanol can be used to convert between mass and volume measurements, enabling the calculation of the volume of a given mass of methanol or vice versa.
-
Buoyancy and Flotation: The lower density of methanol compared to water can be exploited in applications involving the measurement of the density of irregular objects, where methanol can be used as a substitute for water.
-
Solubility and Miscibility: The density of methanol plays a role in determining its solubility and miscibility with other liquids, which is important in various chemical processes and formulations.
-
Thermodynamic Properties: Methanol density is a crucial parameter in the calculation of other thermodynamic properties, such as molar volume, compressibility, and thermal expansion coefficient.
Methanol Density Data and Numerical Examples
To provide a more comprehensive understanding of methanol density, let’s explore some numerical examples and data points:
Methanol Density at Different Temperatures
| Temperature (°C) | Density (g/mL) |
|---|---|
| 0 | 0.8107 |
| 10 | 0.8013 |
| 20 | 0.7918 |
| 30 | 0.7824 |
| 40 | 0.7729 |
| 50 | 0.7635 |
| 60 | 0.7540 |
Methanol Density at Different Pressures
| Pressure (atm) | Density (g/mL) |
|---|---|
| 1 | 0.7918 |
| 5 | 0.7927 |
| 10 | 0.7935 |
| 15 | 0.7943 |
| 20 | 0.7952 |
| 25 | 0.7960 |
| 30 | 0.7968 |
Methanol Density in Solutions
| Methanol Concentration (%) | Density (g/mL) |
|---|---|
| 10 | 0.9818 |
| 20 | 0.9527 |
| 30 | 0.9236 |
| 40 | 0.8945 |
| 50 | 0.9109 |
| 60 | 0.8818 |
| 70 | 0.8527 |
| 80 | 0.8236 |
| 90 | 0.8400 |
| 100 | 0.7918 |
Numerical Examples
- Volume Calculation:
- Given: Mass of methanol = 50 g
- Density of methanol at 20°C = 0.7918 g/mL
-
Volume of methanol = Mass / Density = 50 g / 0.7918 g/mL = 63.14 mL
-
Concentration Determination:
- Given: Density of methanol solution = 0.9109 g/mL
-
From the table, a density of 0.9109 g/mL corresponds to a methanol concentration of 50%.
-
Buoyancy Calculation:
- Given: Mass of an irregular object = 20 g
- Density of water = 1.000 g/mL
- Density of methanol = 0.7918 g/mL
- Buoyant force in water = 20 g × (1.000 g/mL – 0.7918 g/mL) = 4.164 N
- Buoyant force in methanol = 20 g × (0.7918 g/mL – 0.7918 g/mL) = 0 N
- The object will sink in methanol, as the buoyant force is zero.
These examples demonstrate the practical applications of methanol density in various calculations and scenarios.
Conclusion
Methanol density is a crucial physical property that plays a significant role in numerous industrial and scientific applications. Understanding the factors that influence methanol density, as well as the methods used to measure and quantify this parameter, is essential for effectively utilizing this versatile chemical compound. The comprehensive data and numerical examples provided in this guide offer a valuable resource for physicists, chemists, and engineers working with methanol-related systems.
References
- Yao-Nan Wang, et al. “Convenient quantification of methanol concentration detection utilizing an integrated microfluidic chip.” National Pingtung University of Science and Technology, Taiwan.
- “Methanol Concentration – an overview.” ScienceDirect Topics.
- “Processing Method for the Quantification of Methanol and Ethanol Concentration Ranging from 0.195 to 100 g/L.” NCBI.
- “Methanol Permeability – an overview.” ScienceDirect Topics.
- “If methanol (S.G = 0.810) were used rather than water in measuring the density of irregular objects, how would that have affected the result? Explain your answer.” Homework.study.com.

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.