Mercury or hydrargyrum is a transition metal element having a molecular weight of 200.59 g/mol. We will learn more details about Mercury in this article.
Mercury appears as a gray or colorless solid molecule. It is insoluble but reactive with organic protic solvents and soluble in different molten salt but non-reactive to them. It is highly conductive in nature and the value of thermal conductivity of the molecule is 0.125 W/(cm·K) for the solid crystal.
Mercury is diamagnetic in nature and soft material with compressive creep and band gap. Let us discuss the structure, hybridization, polarity, ionic nature, and solubility of Mercury in the following part with proper explanation.
1. How to draw the Mercury structure?
Every molecule have their own specific structure may be those are resembles with some geometric shape or not. let us discuss the structure of mercury molecule.
As there are two same atoms are present so the adopted structure is formed by connecting two mercury atoms. we connect double bonds between them to verify the valency as well as oxidation state of each mercury ion. Double bond is made the structure more strong.
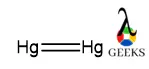
2. Mercury valence electrons
The electrons present in the outer shell of any atom are called valence electrons. Let us count the total valence electrons for Mercury.
The total number of valence electrons present in the outermost shell of Hg2 is 4 where two electrons come from the Hg site and two electrons come from another Hg site because they have only two valence electrons in their outermost shell. So, we just added the individual valence electrons of each atom separately.
- The electronic configuration of Hg is [Xe]4f145d10622
- So, the valence electrons present over the Hg atom is 2, as 6s is the valence orbital of Hg
- So, the total number of valence electrons for the Mercury is 2+2 = 4
3. Mercury structure lone pairs
The lone pairs are those valence electrons present over the valence orbital as remaining after forming the bond. Let us count the total number of lone pairs of Mercury.
The number of lone pairs present over the Mercury molecule is zero because it does not have any lone pairs. The constituent atoms both Hg have only one electron in their valence orbital of them and that one electron is used in the bond formation so, they have zero electrons left.
- The number of lone pairs is calculated by the formula, lone pairs = electrons present in the valence orbital – electrons involved in the bond formation
- The lone pairs present over the Hg atom are, 1-1=0
- So, the total number of lone pairs present over the Mercury molecule is 0+0 = 0
4. Mercury structure shape
The molecular shape of the molecule is an arrangement of the central atom with other atoms in a geometry. Let us predict the molecular shape of the Mercury.
The molecular shape of the Mercury is linear around the central Hg atoms which can be predicted from the following table.
| Molecular Formula |
No. of bond pairs |
No. of lone pairs |
Shape | Geometry |
| 1.AX | 1 | 0 | Linear | Linear |
| 2.AX2 | 2 | 0 | Linear | Linear |
| 3.AXE | 1 | 1 | Linear | Linear |
| 4.AX3 | 3 | 0 | Trigonal planar |
Trigonal Planar |
| 5.AX2E | 2 | 1 | Bent | Trigonal Planar |
| 6.AXE2 | 1 | 2 | Linear | Trigonal Planar |
| 7.AX4 | 4 | 0 | Tetrahedral | Tetrahedral |
| 8.AX3E | 3 | 1 | Trigonal pyramidal |
Tetrahedral |
| 9.AX2E2 | 2 | 2 | Bent | Tetrahedral |
| 10.AXE3 | 1 | 3 | Linear | Tetrahedral |
| 11.AX5 | 5 | 0 | trigonal bipyramidal |
trigonal bipyramidal |
| 12.AX4E | 4 | 1 | seesaw | trigonal bipyramidal |
| 13.AX3E2 | 3 | 2 | t-shaped | trigonal bipyramidal |
| 14.AX2E3 | 2 | 3 | linear | trigonal bipyramidal |
| 15.AX6 | 6 | 0 | octahedral | octahedral |
| 16.AX5E | 5 | 1 | square pyramidal |
octahedral |
| 17.AX4E2 | 4 | 2 | square pyramidal |
octahedral |
The molecular shape of an ionic molecule is determined by the crystal structure and the covalent molecule is predicted by the VSEPR (Valence Shell Electrons Pair Repulsion) theory, and according to this theory, the AX type of molecule having geometry is linear.
5. Mercury structure angle
The bond angle is the angle made by the atoms in a particular shape for proper orientation in that arrangement. Let us calculate the bond angle for the Mercury molecule.
Mercury has linear geometry so it has a bond angle of 1800 because for linear geometry the bond angle is always 1800 from the mathematical calculation. There is no steric repulsion present so there is no chance for deviation of the perfect bond angle for the linear molecule of two Hg atoms.
- Now we merge the theoretical bond angle with the calculated bond angle value by the hybridization value.
- The bond angle formula according to Bent’s rule is COSθ = s/(s-1).
- The Hg is unhybridized but due to linear geometry, it adopts sp hybridization.
- The central atom Hg is sp hybridized, so the s character here is 1/2th
- So, the bond angle is, COSθ = {(1/2)} / {(1/2)-1} =-( 1)
- Θ = COS-1(-1/2) = 1800
6. Mercury structure formal charge
With the help of formal charge, we can predict the partial charge present in a molecule by equal electronegativity. Let us predict the formal charge of Mercury.
The formal charge of Mercury is zero because apparently, it appears neutral, but there is a charge present on the Hg atoms. Those charges are equal in magnitude but opposite in direction, so they can be canceled out and make the molecule neutral.
Let us see how the molecule is neutral on the calculation of formal charge by the formula,
- Formal charge = Nv – Nl.p. -1/2 Nb.p
- The formal charge present over the Hg atom is 1-0-(0/2) = +1
- Th formal charge present over the Hg atom is 0-1-(0/2) = -1
- So, each cation and anion carry one charge and the value is the same but they are opposite in nature and cancel to make the formal charge zero for the Mercury molecule.
7. Mercury hybridization
The central atom undergoes hybridization to form a hybrid orbital of equivalent energy from the atomic orbitals. Let us know about the hybridization of Mercury.
The central Hg is sp hybridized in the Mercury molecule which can be confirmed by the following table.
| Structure | Hybridization value |
State of hybridization of central atom |
Bond angle |
| 1.Linear | 2 | sp /sd / pd | 1800 |
| 2.Planner trigonal |
3 | sp2 | 1200 |
| 3.Tetrahedral | 4 | sd3/ sp3 | 109.50 |
| 4.Trigonal bipyramidal |
5 | sp3d/dsp3 | 900 (axial), 1200(equatorial) |
| 5.Octahedral | 6 | sp3d2/ d2sp3 | 900 |
| 6.Pentagonal bipyramidal |
7 | sp3d3/d3sp3 | 900,720 |
- We can calculate the hybridization by the convention formula, H = 0.5(V+M-C+A),
- So, the hybridization of central Hg is, ½(2+2+0+0) = 2 (sp)
- One s orbital and one p orbital of Hg is involved in the hybridization.
- The lone pairs over the atoms are not involved in the hybridization.
8. Mercury solubility
The most ionic molecule is soluble in water as they can be dissociated. Let us see whether Mercury is soluble in water or not.
Mercury is soluble in water because it can be ionized to form two ions and those ions are soluble in water. When Mercury is dissociated into the ions, it forms Hg+ and this ion can attract the water molecule surrounding by its ionic potential, and the hydride ion can form H-bonding with the water molecule.
Apart from a water molecule, Mercury is soluble in the following solvents
- CCl4
- CS2
- Benzene
- Methanol
- CHCl3
- Ammonia
9. Is Mercury solid or gas?
Ionic compounds are mostly solid in nature because they have a proper crystal structure and strong bonding. Let us check whether Mercury is solid or not.
Mercury is a solid molecule having face center cubic crystal and the energy of the crystal is very strong to stay in solid form. Due to the presence of the crystal, the entropy is very low for the molecule, and for this reason, all the atoms are closely packed in the crystal. It appears as a grey crystalline solid.
10. Is Mercury polar or nonpolar?
Ionic compounds are polar in nature due to the bond formation between them being polar in character. Let us check whether the Mercury molecule is polar or not.
Mercury is a non-polar molecule because there is zero electronegativity difference present in two atoms as both are the same element.
11. Is Mercury acidic or basic?
If a molecule can release a proton or hydroxide ions in an aqueous solution then it is called acid or base respectively. Let us check whether Mercury is basic or not.
Mercury is a strong base although it does not have H+ or OH– ions. It has a hydride ion that can draw the proton from other subsequent and form conjugate acid. Hydride ion has a higher affinity to draw the proton to form a hydrogen molecule and behaves as a strong bronsted base.
12. Is Mercury electrolyte?
Ionic molecules have higher electrolytic nature because they are formed by the strong interaction of ions. Let us see whether Mercury is an electrolyte or not.
Mercury is a strong electrolyte because when it dissociates into an aqueous solution it formed Hg+, which are strong ions and the mobility of those ions is very high. The ionic potential of those ions are very higher and carry electricity through the aqueous solution very fast.
13. Is Mercury ionic or covalent?
The ionic molecule has strong interaction between constituent atoms and has higher polarizing power. Let us see if Mercury is ionic or not.
Mercury is an ionic molecule because the molecule is formed by the electron donation and acceptance mechanism not by sharing. Also, Hg+ has higher ionic potential due to charge density so it can polarize the anion easily and Hg ion has greater polarizability according to Fajan’s rule it is an ionic molecule.
14. Is mercury a denaturing agent?
The chemical compounds or reagents which can break the peptide bonds or protein structure are called denaturing agent. let us see whether mercury is denaturing agent or not.
Mercury is a denaturing agent because it can break the primary as well as secondary protein structure. due to hydrophobic nature of the mercury it can not composed the peptide bond, it easily cleaved the amino group of 2nd protein and acid group of former protein. But methyl mercury is reversible denaturing agent.
Conclusion
Mercury is a strong inorganic Bronsted base and it can be used in many organic reactions to pull out the acidic proton from the desired molecule. Due to fully filled d orbital it is behaves differently from other transition elements.

Hi……I am Biswarup Chandra Dey, I have completed my Master’s in Chemistry from the Central University of Punjab. My area of specialization is Inorganic Chemistry. Chemistry is not all about reading line by line and memorizing, it is a concept to understand in an easy way and here I am sharing with you the concept about chemistry which I learn because knowledge is worth to share it.