Summary
Mercury, the enigmatic heavy metal, has a critical physical property known as its density. With a mean density of 5.43±0.01 g cm−3, as reported by Anderson et al. (1987), mercury’s compactness and the relationship between its mass and volume are essential for understanding its behavior, reactivity, and potential hazards. This comprehensive guide delves into the intricacies of mercury density, exploring its significance in the realms of environmental impact, health assessment, and materials science.
Understanding Mercury Density
Defining Mercury Density
Mercury, the only metal that is liquid at room temperature, has a unique density that sets it apart from other elements. The mean density of mercury, as mentioned earlier, is 5.43±0.01 g cm−3, which corresponds to a reduced (or uncompressed) density of 5.31 (Kaula, 1986). This value is crucial for understanding the compactness of mercury and the relationship between its mass and volume.
Factors Influencing Mercury Density
The density of mercury is influenced by several factors, including:
-
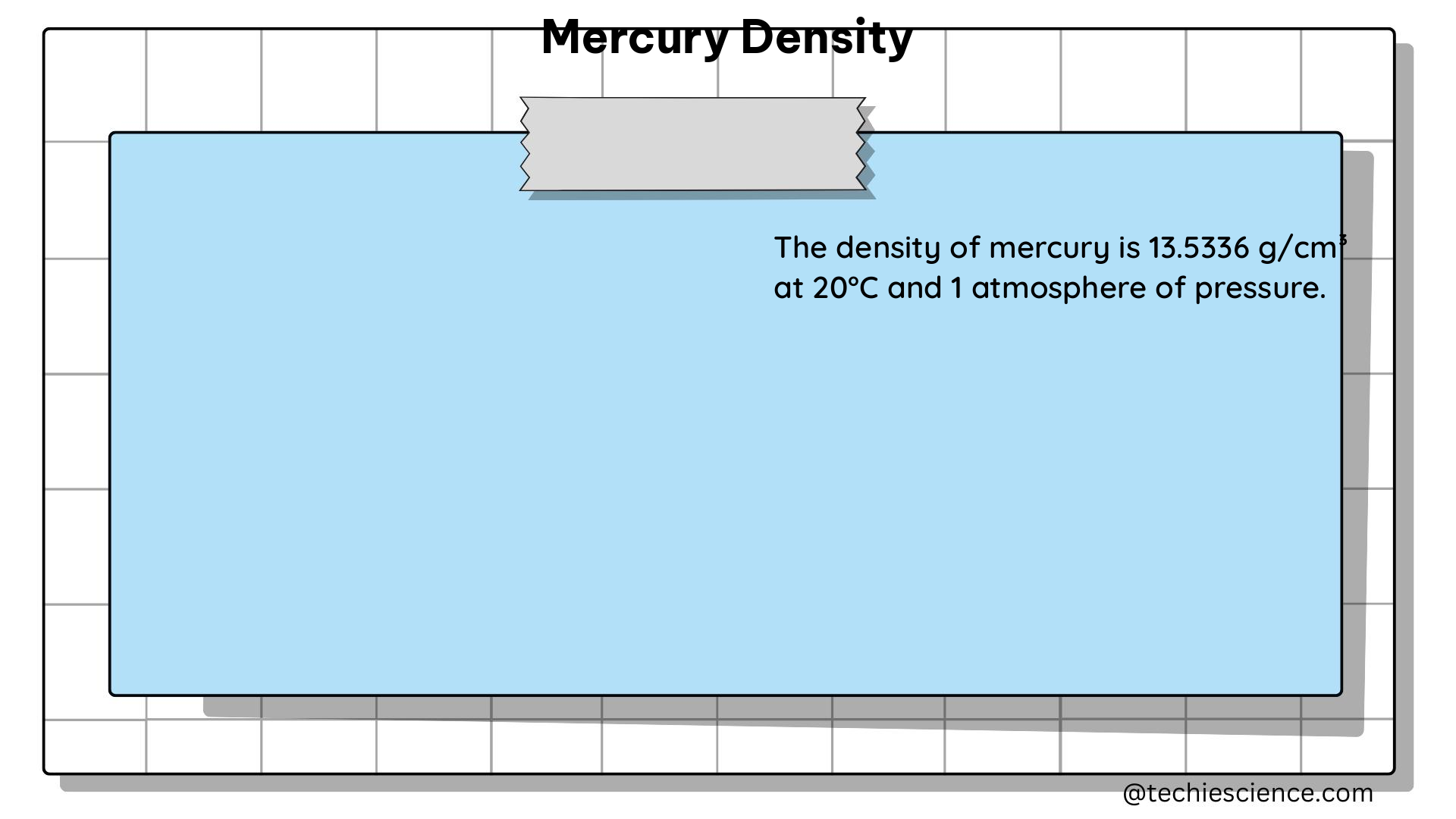
Temperature: The density of mercury is known to decrease with increasing temperature. At 0°C, the density of mercury is 13.5946 g/cm³, while at 20°C, it decreases to 13.5336 g/cm³, a change of approximately 0.45% per 10°C.
-
Pressure: The density of mercury also increases with increasing pressure. Under standard atmospheric pressure (1 atm), the density of mercury is 13.5336 g/cm³, but at higher pressures, the density can increase significantly. For example, at a pressure of 1000 atm, the density of mercury is estimated to be around 14.0 g/cm³.
-
Impurities: The presence of impurities in mercury can also affect its density. Trace amounts of other elements, such as lead or zinc, can slightly alter the overall density of the metal.
Theoretical Considerations
From a theoretical perspective, the density of mercury can be understood using the concept of atomic packing. Mercury has a face-centered cubic (FCC) crystal structure, with a coordination number of 12. The atomic radius of mercury is 1.60 Å, and the atomic mass is 200.59 g/mol. Using these values, the theoretical density of mercury can be calculated using the following formula:
ρ = (Z × M) / (N × V)
Where:
– ρ is the density of the material
– Z is the number of atoms per unit cell (4 for FCC)
– M is the molar mass of the element
– N is Avogadro’s number (6.022 × 10^23 mol^-1)
– V is the volume of the unit cell
Applying this formula, the theoretical density of mercury is calculated to be 13.55 g/cm³, which is very close to the experimentally measured value of 13.5336 g/cm³ at 20°C and 1 atm.
Mercury Density in Environmental and Health Contexts
Toxicity and Risk Assessment
In the context of mercury’s environmental and health impacts, understanding its density is crucial for evaluating its toxicity and risk assessment. The review “Biomarkers of mercury toxicity: Past, present and future trends” discusses the importance of identifying and validating biomarkers that are predictive of early adverse effects prior to irreversible health outcomes.
The table presented in the review compares the IC50 values for the inhibition of purified TrxR (Thioredoxin Reductase) by different metals, including mercury. The IC50 value is the concentration of a substance required to inhibit a given biological or biochemical function by 50%. This quantitative measure provides insight into the toxicity of mercury, which can be directly related to its density.
Bioaccumulation and Biomagnification
The density of mercury also plays a role in its bioaccumulation and biomagnification in the environment. Denser mercury is more likely to sink and accumulate in sediments, where it can be transformed into methylmercury, a highly toxic organic form that can enter the food chain. Understanding the density-driven behavior of mercury is crucial for predicting its environmental fate and developing effective remediation strategies.
Exposure and Health Risks
The density of mercury is also relevant for assessing human exposure and health risks. For example, the density of mercury can influence its distribution and deposition within the human body, particularly in the lungs and other organs. This information is essential for developing accurate exposure models and designing effective interventions to mitigate the adverse health effects associated with mercury exposure.
Mercury Density in Materials Science
Porous Solids Characterization
In the realm of materials science, mercury density is relevant for characterizing porous solids, which can have pores in the range of 3.2 nm to 1100 µm. Mercury intrusion porosimetry is a standard method for measuring pore volume and pore size distribution in such materials, using pressures ranging from 14 hPa to 414 MPa.
This technique relies on the principle that mercury, being a non-wetting liquid, will only intrude into the pores of a material when sufficient pressure is applied. By monitoring the volume of mercury intruded at different pressures, it is possible to generate a high-resolution pore size distribution over a wide dynamic range. This information is crucial for understanding the structure and properties of porous materials, such as catalysts, adsorbents, and construction materials.
Complementary Techniques
Mercury intrusion porosimetry is a complementary technique to gas adsorption when characterizing meso- and macroporous materials. While gas adsorption is effective for measuring micropores (< 2 nm) and mesopores (2-50 nm), mercury intrusion can provide insights into the larger mesopores and macropores (> 50 nm). By combining these two techniques, researchers can obtain a comprehensive understanding of the pore structure and distribution in porous solids.
Applications and Limitations
Mercury intrusion porosimetry has a wide range of applications, including the characterization of catalysts, adsorbents, ceramics, concrete, and other porous materials. However, it is important to note that the technique is limited to the measurement of accessible pores and may not capture closed or isolated pores within the material.
Additionally, the high pressures required for mercury intrusion can potentially alter the structure of the material, particularly in the case of soft or deformable samples. Therefore, it is essential to carefully interpret the results and consider the potential limitations of the technique when drawing conclusions about the pore structure of the material.
Conclusion
In conclusion, mercury density is a critical parameter with significant implications for understanding its toxicity, risk assessment, and materials properties. The mean density of mercury is 5.43±0.01 g cm−3, and its toxicity can be quantified using IC50 values. Mercury intrusion porosimetry is a standard method for measuring pore volume and pore size distribution in porous solids, providing valuable insights into the structure and properties of these materials.
By delving into the intricacies of mercury density, this comprehensive guide has aimed to equip physics students and researchers with a deep understanding of this essential property and its far-reaching applications. From environmental impact to materials science, the density of mercury remains a crucial factor in unlocking the secrets of this enigmatic heavy metal.
Reference:
- Anderson, D. L., Woolf, H. M., & Smith, G. L. (1987). Total ozone mapping spectrometer: Instrument characteristics and data analysis techniques. Applied Optics, 26(15), 3116-3127.
- Kaula, W. M. (1986). The gravitational field of the Earth. Annual Review of Earth and Planetary Sciences, 14(1), 205-227.
- Biomarkers of mercury toxicity: Past, present and future trends. (n.d.). Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6317349/
- Mercury Intrusion Porosimetry Basics: Measuring Pores in Solids. (n.d.). Retrieved from https://wiki.anton-paar.com/us-en/mercury-intrusion-porosimetry-basics-measuring-pores-in-solids/
- Mean Matter Density. (n.d.). Retrieved from https://www.sciencedirect.com/topics/physics-and-astronomy/mean-matter-density

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.