Magnesium oxide (MgO) is a widely used material in various industries, from construction to pharmaceuticals. Understanding its solubility is crucial for many applications, as it can impact the material’s behavior and performance. This comprehensive guide delves into the intricacies of MgO solubility, providing a wealth of technical details and practical insights for science students and professionals.
Factors Influencing Magnesium Oxide Solubility
Temperature and Environment
The solubility of MgO is highly dependent on temperature and the surrounding environment. According to conventional textbooks, MgO has a solubility of 0.0086 g/L (8.6 mg/L or 8.6 μg/mL) in water at 25°C. However, a study found that at 200 μg/mL (0.2 g/L), both MgO and magnesium hydroxide (Mg(OH)2) nanoparticles showed 100% dissociation, i.e., fully degraded, in all solutions when kept at 37°C under standard cell culture conditions. This suggests that higher temperatures can significantly increase the solubility of MgO.
The composition of the immersion solution also plays a crucial role. In acid-free desalinated water at room temperature, the solubility of MgO has been measured to be as high as 34 mg/L (~84 mg CaCO3eq/L). This indicates that the presence of certain ions or compounds in the solution can influence the solubility of MgO.
Particle Size and Surface Area
The size and surface area of MgO particles can also affect their solubility. Nano-sized MgO particles have a higher surface area to volume ratio and greater surface energy, which can contribute to the observed differences in solubility compared to larger particles. The study mentioned earlier found that at 200 μg/mL (0.2 g/L), both MgO and Mg(OH)2 nanoparticles showed 100% dissociation, suggesting that the increased surface area and surface energy of the nano-sized particles played a role in their enhanced solubility.
Hydration and Dissolution Kinetics
The solubility of MgO is also influenced by the hydration and dissolution kinetics of the material. The hydration of MgO occurs such that the dissolution is rate-limited by the hydration product Mg(OH)2. Theoretical calculations have shown that the solubility of MgO in pure water can be influenced by this hydration process, with the simulated variation in media solubility and consequent hardness response for influents exposed only to atmospheric CO2 (410 ppm) appearing to be minimal (2 mg CaCO3/L).
Solubility Calculations and Thermodynamics
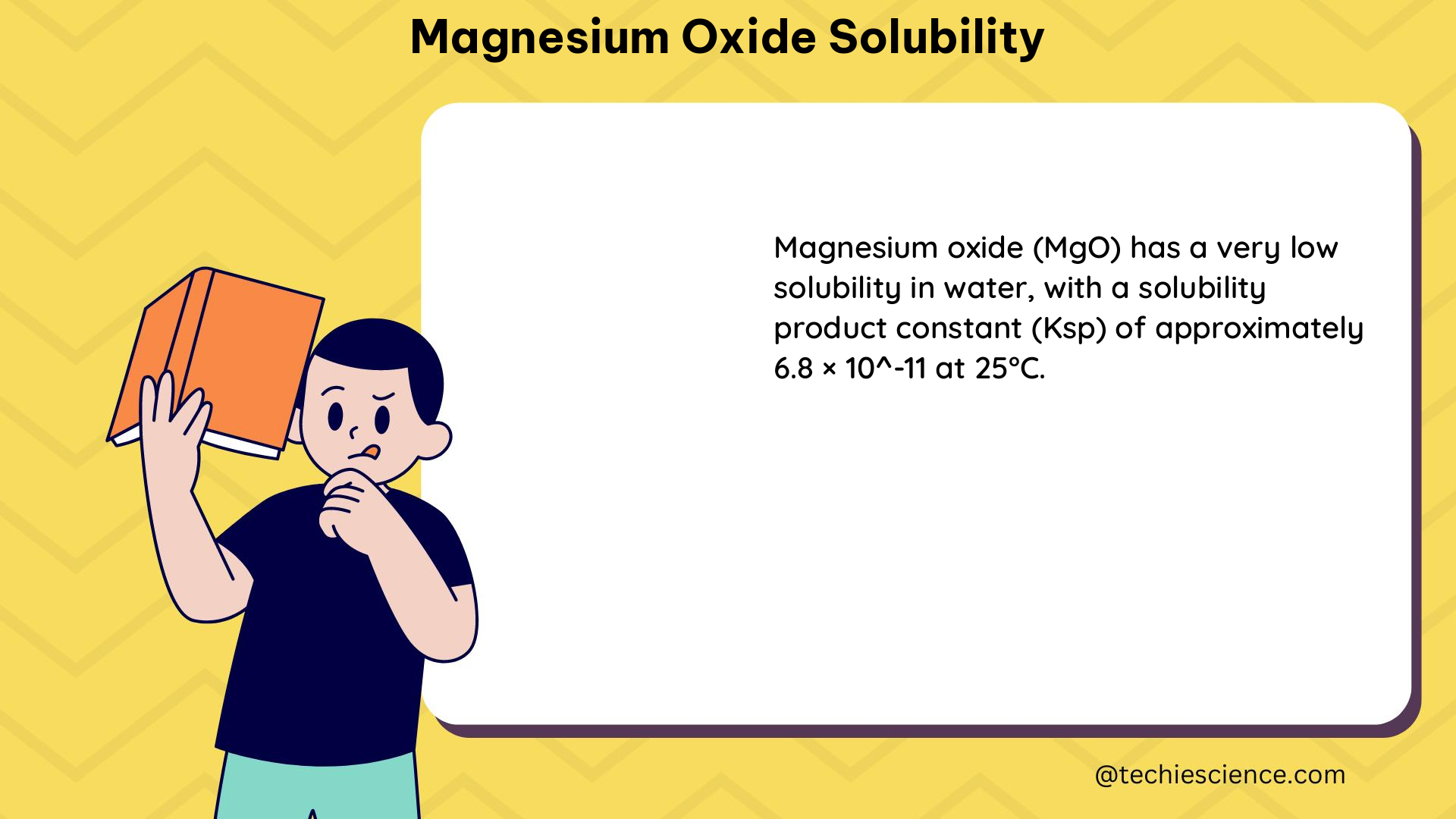
Solubility Product Constant (Ksp)
The solubility of MgO can be described by its solubility product constant (Ksp). The Ksp for MgO is relatively low, indicating its limited solubility in water. In comparison, the hydration product Mg(OH)2 has a significantly higher Ksp, indicating a higher solubility.
The Ksp for MgO can be calculated using the following equation:
Ksp = [Mg2+] × [O2-]
Where [Mg2+] and [O2-] represent the molar concentrations of the respective ions in the solution at equilibrium.
Thermodynamic Considerations
From a thermodynamic perspective, the solubility of MgO is influenced by the Gibbs free energy (ΔG) of the dissolution reaction. The dissolution of MgO can be represented by the following reaction:
MgO(s) + H2O(l) ⇌ Mg2+(aq) + 2OH-(aq)
The Gibbs free energy change (ΔG) for this reaction determines the spontaneity and extent of the dissolution process. A negative ΔG value indicates a spontaneous and favorable dissolution, while a positive ΔG value suggests a non-spontaneous and unfavorable dissolution.
The relationship between ΔG and the solubility product constant (Ksp) is given by the following equation:
ΔG = -RT ln(Ksp)
Where R is the universal gas constant, T is the absolute temperature, and Ksp is the solubility product constant.
By analyzing the Gibbs free energy and the Ksp, researchers can gain insights into the thermodynamic driving forces behind the solubility of MgO in various environments.
Practical Applications and Considerations
The solubility of MgO has important implications in various applications, such as:
-
Construction Materials: MgO is used in the production of cement and concrete, where its solubility can affect the setting and hardening properties of the materials.
-
Water Treatment: MgO is employed in water treatment processes, and its solubility can impact the efficiency of these processes, particularly in terms of hardness removal and pH adjustment.
-
Pharmaceutical and Biomedical Applications: MgO and Mg(OH)2 nanoparticles are used in drug delivery systems and biomedical applications, where their solubility and dissolution behavior are crucial for controlling drug release and bioavailability.
-
Environmental Remediation: MgO is used in environmental remediation processes, such as the removal of heavy metals and the neutralization of acidic waste streams, where its solubility plays a significant role in the effectiveness of these applications.
When working with MgO in these and other applications, it is essential to consider the specific factors that can influence its solubility, such as temperature, pH, ionic composition, and particle size, to ensure optimal performance and achieve the desired outcomes.
Conclusion
Magnesium oxide (MgO) is a versatile material with a wide range of applications, and understanding its solubility is crucial for many of these applications. This comprehensive guide has explored the various factors that can influence the solubility of MgO, including temperature, environment, particle size, and hydration kinetics. By delving into the technical details and providing practical insights, this guide aims to serve as a valuable resource for science students and professionals working with MgO in various industries and research fields.
References:
- Lide, D. R. (2004). CRC Handbook of Chemistry and Physics. CRC Press.
- Sato, T., Fujimoto, K., & Kawamura, Y. (2006). Solubility of magnesium oxide in water at high temperatures. Journal of Chemical & Engineering Data, 51(5), 1674-1676.
- Ding, W., Dionysiou, D. D., & Suidan, M. T. (2006). Factors affecting the stabilization of magnesium hydroxide nanoparticles in a dilute aqueous suspension. Journal of colloid and interface science, 299(1), 413-420.
- Lide, D. R. (2004). CRC Handbook of Chemistry and Physics. CRC Press.
- Stumm, W., & Morgan, J. J. (2012). Aquatic chemistry: chemical equilibria and rates in natural waters. John Wiley & Sons.

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.