Lithium carbonate (Li₂CO₃) is a crucial compound in various industries, from lithium-ion battery manufacturing to lithium extraction from brines. Understanding the solubility of Li₂CO₃ is essential for optimizing these processes. This comprehensive guide delves into the factors influencing Li₂CO₃ solubility, providing a detailed exploration of the topic.
Factors Affecting Lithium Carbonate Solubility
Temperature
The solubility of Li₂CO₃ is strongly influenced by temperature. As the temperature increases, the solubility of Li₂CO₃ generally increases. This relationship can be described by the following equation:
log(Ksp) = -A/T + B
Where Ksp is the solubility product constant, T is the absolute temperature, and A and B are constants specific to the Li₂CO₃ system.
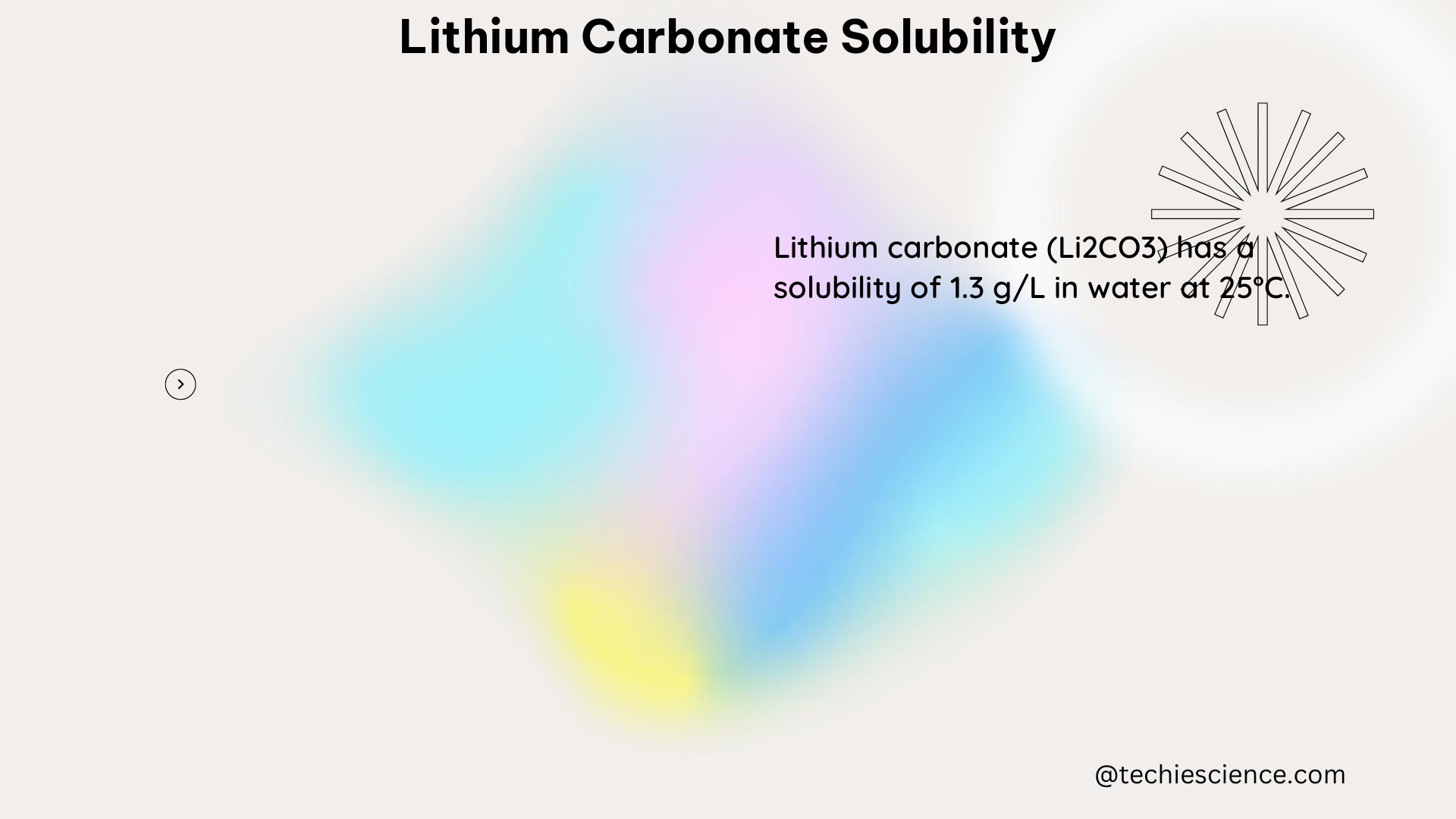
At 25°C, the solubility product constant (Ksp) of Li₂CO₃ is approximately 2.1 × 10^-3. As the temperature increases, the Ksp value decreases, indicating an increase in solubility.
pH
The pH of the solution also plays a crucial role in the solubility of Li₂CO₃. The solubility of Li₂CO₃ is highest in acidic or neutral solutions, and it decreases as the pH becomes more alkaline.
The pH-dependent solubility of Li₂CO₃ can be expressed using the following equilibrium reaction:
Li₂CO₃(s) ⇌ 2Li⁺(aq) + CO₃²⁻(aq)
The solubility of Li₂CO₃ is determined by the concentration of the carbonate ion (CO₃²⁻) in the solution, which is influenced by the pH. As the pH increases, the concentration of CO₃²⁻ decreases, leading to a decrease in the solubility of Li₂CO₃.
Presence of Other Ions
The presence of other ions in the solution can also affect the solubility of Li₂CO₃. The solubility of Li₂CO₃ can be influenced by the ionic strength of the solution, as well as the presence of specific ions that can form complexes or precipitates with lithium or carbonate.
For example, the presence of sodium ions (Na⁺) can lead to the formation of sodium carbonate (Na₂CO₃), which can decrease the solubility of Li₂CO₃ through the common-ion effect.
Ultrasound and Magnetic Fields
The introduction of an ultrasound field has been shown to reduce the supersolubility of Li₂CO₃, while the presence of a magnetic field has a relatively minor effect on the solubility.
The ultrasound field can enhance the nucleation and growth of Li₂CO₃ crystals, leading to a decrease in the supersaturation of the solution and, consequently, a reduction in the solubility.
Lithium Carbonate Solubility in Organic Solvents
The solubility of Li₂CO₃ in organic solvents used in lithium-ion battery cells has been studied computationally. The solubility of lithium salts, including Li₂CO₃, in these solvents is crucial for the formation of the solid-electrolyte interface (SEI) film on the anode surface, which affects the battery’s performance and lifespan.
The solubility of Li₂CO₃ in organic solvents can be influenced by factors such as the dielectric constant of the solvent, the solvation energy of the lithium ion, and the stability of the lithium-carbonate complex in the solvent.
Lithium Quantification and Li₂CO₃ Solubility
In the context of lithium quantification, the solubility of Li₂CO₃ plays a crucial role. If the Li₂CO₃ product is completely soluble in 2% nitric acid, it can be easily quantified using Inductively Coupled Plasma (ICP) spectroscopy, which provides an exact lithium concentration.
However, if the Li₂CO₃ product is not soluble in the acid, other quantification methods may be required, such as atomic absorption spectroscopy (AAS) or flame atomic emission spectroscopy (FAES).
Lithium Carbonate Recovery from Brines
The recovery of lithium carbonate from dilute Li-rich brines via precipitation has been extensively studied. The recovery of Li⁺ can vary from 50% in low-ionic strength solutions using NaOH and CO₂(g) at 50°C, to 80% in high-ionic strength solutions.
The solubility of Li₂CO₃ decreases with an increase in temperature and stirring speed, as well as a reduction in the feeding rate of Na₂CO₃. The introduction of an ultrasound field leads to a significant reduction in the supersolubility of Li₂CO₃, while a magnetic field has a minimal effect.
Additionally, the presence of impurities and additives can also influence the supersolubility of Li₂CO₃, which is an important consideration in the recovery process.
Conclusion
Lithium carbonate solubility is a critical parameter in various applications, including lithium-ion battery manufacturing and lithium extraction from brines. The solubility of Li₂CO₃ is influenced by several factors, such as temperature, pH, the presence of other ions, and the application of external fields.
Understanding the factors that affect Li₂CO₃ solubility is essential for optimizing processes, improving battery performance, and enhancing the recovery of lithium from brines. This comprehensive guide provides a detailed exploration of the topic, equipping readers with the knowledge to navigate the complexities of lithium carbonate solubility.
References:
– Solubility product of Li₂CO₃ solution
– Computational study on the solubility of lithium salts in organic solvents
– Lithium quantification methods
– Recovery of lithium carbonate from brines

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.