Lead chloride (PbCl₂) is a chemical compound that has been extensively studied due to its importance in various industrial and environmental applications. Understanding the solubility of lead chloride is crucial for predicting its behavior in chemical reactions, environmental processes, and industrial processes. This comprehensive guide will delve into the technical details of lead chloride solubility, providing a valuable resource for science students and professionals.
Introduction to Lead Chloride Solubility
Lead chloride is a white, crystalline solid that is moderately soluble in water. The solubility of lead chloride is influenced by various factors, including temperature, pH, and the presence of other ions in the solution. The aqueous solubility of lead chloride is an important parameter in understanding its behavior in water-based systems, such as water treatment, soil remediation, and environmental monitoring.
Solubility of Lead Chloride in Pure Water
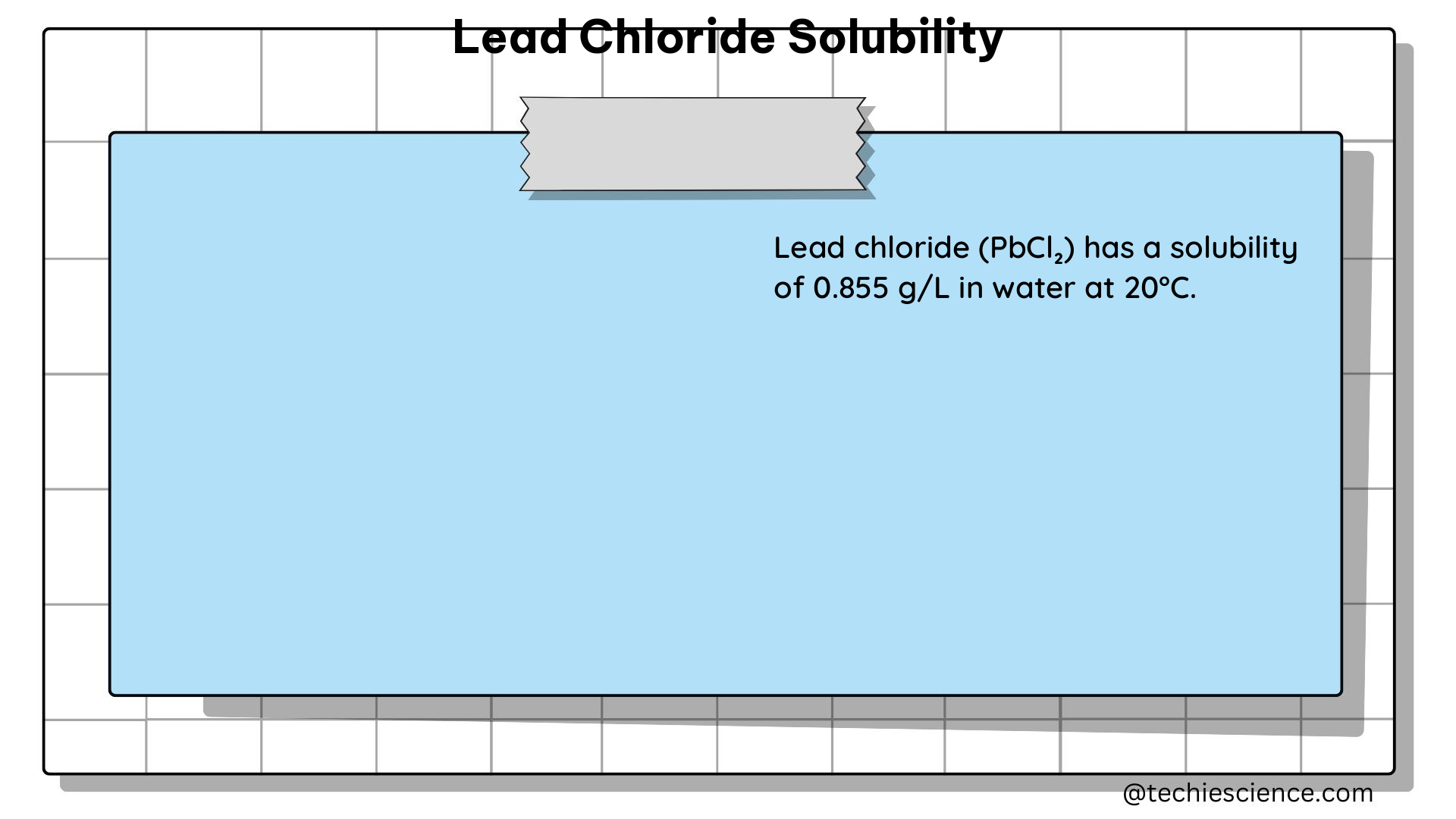
The solubility of lead chloride in pure water has been measured to be 4.44 g/L at 25°C. This value represents the maximum concentration of lead chloride that can be dissolved in pure water under standard conditions. The molar solubility of lead chloride can be calculated using the following equation:
Molar Solubility = Solubility (g/L) / Molar Mass (g/mol)
The molar mass of lead chloride is 278.1 g/mol, so the molar solubility of lead chloride in pure water can be calculated as:
Molar Solubility = 4.44 g/L / 278.1 g/mol = 0.0159 M
This molar solubility value represents the maximum concentration of dissolved lead and chloride ions in the solution at equilibrium.
Solubility Product Constant (Ksp) of Lead Chloride
The solubility of lead chloride in water can also be expressed in terms of its solubility product constant (Ksp). The Ksp for lead chloride is 1.7 × 10⁻⁵ at 25°C. The Ksp is a thermodynamic constant that describes the equilibrium between the dissolved ions and the solid phase of a sparingly soluble salt.
The Ksp for lead chloride can be expressed as:
Ksp = [Pb²⁺][Cl⁻]²
Where [Pb²⁺] and [Cl⁻] represent the molar concentrations of the lead and chloride ions, respectively, at equilibrium.
Using the Ksp value and the molar solubility of lead chloride, we can verify the calculated molar solubility:
Ksp = [Pb²⁺][Cl⁻]² = (0.0159 M)²(0.0159 M)² = 1.7 × 10⁻⁵
This calculation confirms that the molar solubility of 0.0159 M is consistent with the reported Ksp value for lead chloride.
Factors Affecting Lead Chloride Solubility
The solubility of lead chloride can be influenced by several factors, including:
-
Temperature: The solubility of lead chloride generally increases with increasing temperature. This is due to the endothermic nature of the dissolution process, where the dissolution of lead chloride is favored at higher temperatures.
-
pH: The solubility of lead chloride is affected by the pH of the solution. At lower pH values (more acidic conditions), the solubility of lead chloride increases due to the formation of soluble lead-containing species, such as Pb²⁺ and PbCl⁺.
-
Presence of other ions: The presence of other ions in the solution, such as sulfate (SO₄²⁻) or carbonate (CO₃²⁻), can affect the solubility of lead chloride. These ions can form insoluble lead-containing compounds, which can decrease the overall solubility of lead chloride.
-
Complexation: The formation of soluble lead-containing complexes, such as PbCl₃⁻ or PbCl₄²⁻, can increase the apparent solubility of lead chloride in the solution.
Solubility Equilibrium and Precipitation of Lead Chloride
The solubility of lead chloride in water is governed by a solubility equilibrium, which can be represented by the following equation:
PbCl₂(s) ⇌ Pb²⁺(aq) + 2Cl⁻(aq)
At equilibrium, the concentrations of the dissolved lead and chloride ions are related to the solubility product constant (Ksp) through the following expression:
Ksp = [Pb²⁺][Cl⁻]²
If the ion product, [Pb²⁺][Cl⁻]², exceeds the Ksp value, the solution becomes supersaturated, and lead chloride will precipitate out of the solution. Conversely, if the ion product is less than the Ksp value, the solution is undersaturated, and lead chloride will continue to dissolve.
The precipitation of lead chloride can be represented by the reverse of the solubility equilibrium equation:
Pb²⁺(aq) + 2Cl⁻(aq) → PbCl₂(s)
This precipitation process is important in various applications, such as water treatment, where lead chloride can be removed from the solution by precipitation.
Numerical Examples and Calculations
- Calculating the Molar Solubility of Lead Chloride
Given:
– Solubility of lead chloride in pure water = 4.44 g/L
– Molar mass of lead chloride = 278.1 g/mol
Molar Solubility = Solubility (g/L) / Molar Mass (g/mol)
Molar Solubility = 4.44 g/L / 278.1 g/mol = 0.0159 M
- Verifying the Solubility Product Constant (Ksp) of Lead Chloride
Given:
– Molar Solubility of lead chloride = 0.0159 M
– Ksp for lead chloride = 1.7 × 10⁻⁵
Ksp = [Pb²⁺][Cl⁻]²
Ksp = (0.0159 M)²(0.0159 M)² = 1.7 × 10⁻⁵
The calculated Ksp value matches the reported value, confirming the consistency of the molar solubility and Ksp for lead chloride.
- Determining the Precipitation Conditions for Lead Chloride
Suppose the ion product, [Pb²⁺][Cl⁻]², is greater than the Ksp value of 1.7 × 10⁻⁵. In this case, the solution would be supersaturated, and lead chloride would precipitate out of the solution.
Conversely, if the ion product is less than the Ksp value, the solution would be undersaturated, and lead chloride would continue to dissolve.
Conclusion
Lead chloride solubility is a crucial parameter in understanding the behavior of this compound in various chemical and environmental systems. This comprehensive guide has provided detailed information on the solubility of lead chloride in pure water, the solubility product constant, and the factors that influence its solubility. The numerical examples and calculations demonstrate the practical application of these concepts. By understanding the intricacies of lead chloride solubility, science students and professionals can better predict and control the behavior of this important chemical compound.
References
- PubChem. (n.d.). Lead chloride. Retrieved from https://pubchem.ncbi.nlm.nih.gov/compound/Lead-chloride
- ScienceDirect. (n.d.). Lead chloride. Retrieved from https://www.sciencedirect.com/topics/medicine-and-dentistry/lead-chloride
- Homework.study.com. (n.d.). Lead chloride dissolves in water according to PbCl2(s) ⇌ Pb2+(aq) + 2Cl-(aq). The solubility in pure water has been measured to be 4.44 g/L. Calculate the solubility product of lead chloride in pure water. Retrieved from https://homework.study.com/explanation/lead-chloride-dissolves-in-water-according-to-pbcl2-s-arrow-pb2-plus-plus-2cl-aq-the-solubility-in-pure-water-has-been-measured-to-be-4-44-gl-1-calculate-the-solubility-product-of-lead-chloride-in-pure.html

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.