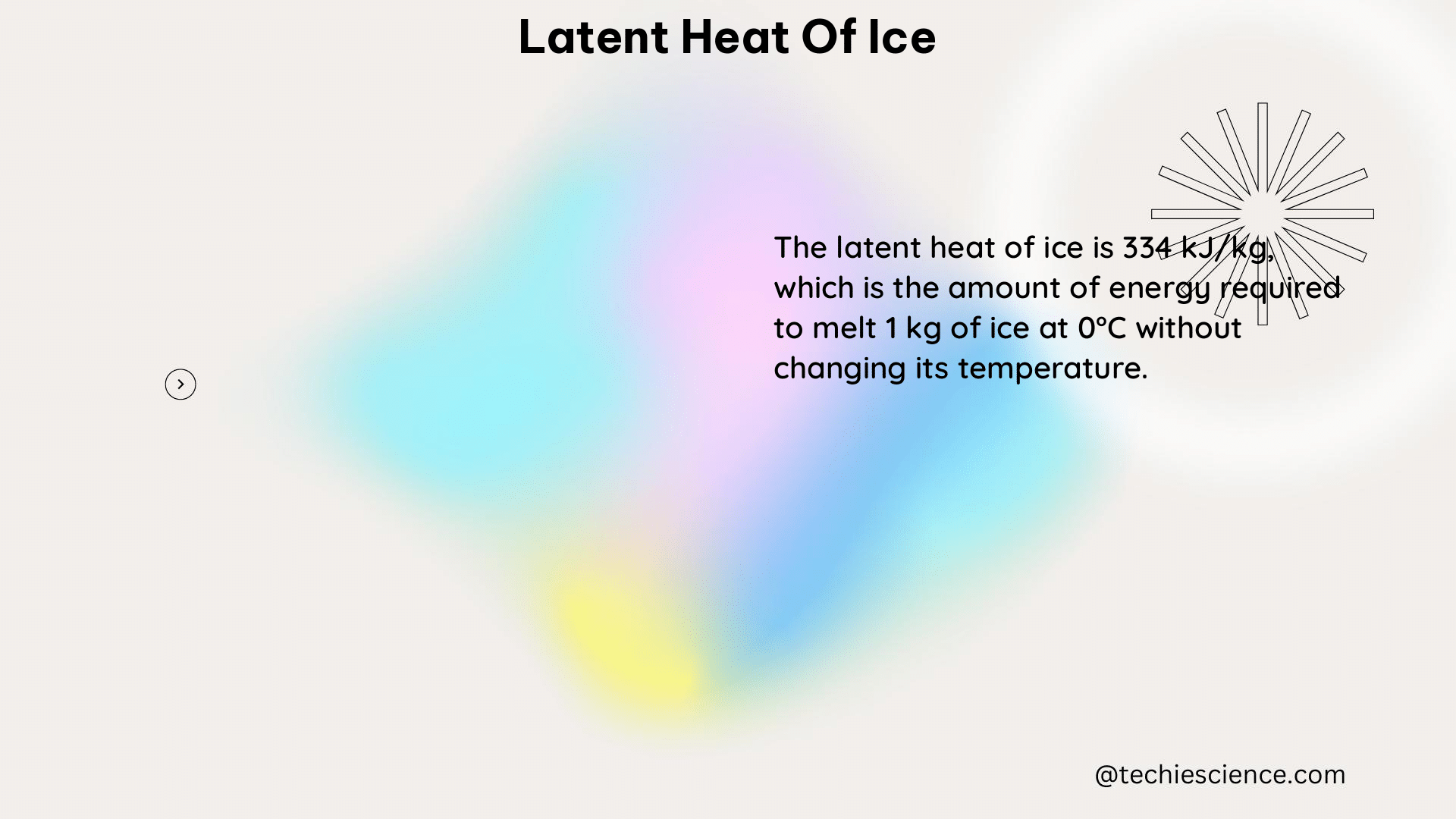
The latent heat of ice, also known as the latent heat of fusion of ice, is the amount of heat energy required to change one gram of ice at 0°C into water at 0°C without a change in temperature. The accepted value for the latent heat of fusion of ice is 333.5 J/g.
Understanding the Latent Heat of Fusion of Ice
The latent heat of fusion is the energy required to change a substance from a solid state to a liquid state without changing its temperature. In the case of ice, the latent heat of fusion is the energy required to melt ice at 0°C into liquid water at 0°C.
The formula for calculating the latent heat of fusion (Lf) is:
Lf = q/m
Where:
– q is the heat absorbed or released during the phase change (in Joules)
– m is the mass of the substance (in grams)
For ice, the accepted value for the latent heat of fusion is 333.5 J/g, meaning that 333.5 Joules of energy are required to melt 1 gram of ice at 0°C into 1 gram of water at 0°C.
Measuring the Latent Heat of Fusion of Ice
There are several methods that can be used to measure the latent heat of fusion of ice. Here are two common methods:
Method 1: Using a Calorimeter
- Prepare the Calorimeter: Fill the calorimeter with a known mass of warm water (around 40°C) and measure its initial temperature.
- Add Ice: Add a known mass of ice (made from distilled water) to the warm water and stir gently until the ice is completely melted.
- Measure the Final Temperature: Measure the final temperature of the water-ice mixture.
- Calculate the Heat Absorbed: Use the formula q = mcΔT, where:
- q is the heat absorbed by the ice (in Joules)
- m is the mass of the ice (in grams)
- c is the specific heat capacity of ice (2.09 J/g°C)
- ΔT is the change in temperature (in °C)
- Calculate the Latent Heat of Fusion: Use the formula Lf = q/m to calculate the latent heat of fusion of ice.
Method 2: Using an Electric Heater and Ice Container
- Prepare the Ice Container: Weigh an ice container with a known mass.
- Heat the Ice: Immerse an electric heater in the ice container and turn it on. Record the power of the heater (in Watts) and the time (in seconds) it takes to melt the ice.
- Calculate the Heat Gained by the Ice: Use the formula H = mc(T2 – T1), where:
- H is the heat energy gained by the ice (in Joules)
- m is the mass of the ice (in kg)
- c is the specific heat capacity of ice (2.09 J/kg°C)
- T2 is the final temperature of the ice (in °C)
- T1 is the initial temperature of the ice (in °C)
- Calculate the Latent Heat of Fusion: Use the formula Lf = Pt/m, where:
- Lf is the latent heat of fusion of ice (in J/g)
- P is the power of the heater (in Watts)
- t is the time taken to melt the ice (in seconds)
- m is the mass of the ice (in grams)
Factors Affecting the Measurement of Latent Heat of Fusion
When measuring the latent heat of fusion of ice, it is important to consider the following factors:
- Purity of Ice: The latent heat of fusion of pure ice is 333.5 J/g, but the presence of impurities can lower this value. It is recommended to use distilled water to make the ice.
- Water Temperature: The temperature of the water used should be above the freezing point of water but not too hot, typically around 40°C or less, to ensure a small temperature difference between the water and the ice.
- Heat Loss to Surroundings: Minimizing heat loss to the surroundings is crucial for accurate measurements. Using an insulated container, such as a styrofoam cup, can help reduce heat loss.
- Number of Trials: Performing the experiment multiple times and taking the average of the results can help reduce the impact of errors and uncertainties in the measurement.
Numerical Examples
- Example 1: If 50 g of ice at 0°C is added to 200 g of water at 40°C, and the final temperature of the mixture is 10°C, calculate the latent heat of fusion of ice.
Given:
– Mass of ice (m) = 50 g
– Initial temperature of water (T1) = 40°C
– Final temperature of the mixture (T2) = 10°C
– Specific heat capacity of water (c) = 4.18 J/g°C
Step 1: Calculate the heat absorbed by the ice.
Heat absorbed by the ice = m × c × (T2 – 0)
Heat absorbed by the ice = 50 g × 2.09 J/g°C × (10°C – 0°C)
Heat absorbed by the ice = 1045 J
Step 2: Calculate the latent heat of fusion of ice.
Latent heat of fusion of ice (Lf) = Heat absorbed by the ice / Mass of ice
Lf = 1045 J / 50 g
Lf = 20.9 J/g
- Example 2: An electric heater with a power of 100 W is used to melt 20 g of ice at 0°C. If the time taken to melt the ice is 120 seconds, calculate the latent heat of fusion of ice.
Given:
– Power of the heater (P) = 100 W
– Time taken to melt the ice (t) = 120 s
– Mass of ice (m) = 20 g
Step 1: Calculate the heat energy supplied by the heater.
Heat energy supplied = Power × Time
Heat energy supplied = 100 W × 120 s
Heat energy supplied = 12,000 J
Step 2: Calculate the latent heat of fusion of ice.
Latent heat of fusion of ice (Lf) = Heat energy supplied / Mass of ice
Lf = 12,000 J / 20 g
Lf = 600 J/g
These examples demonstrate the application of the formulas and principles discussed earlier to calculate the latent heat of fusion of ice.
Conclusion
The latent heat of fusion of ice is a fundamental concept in thermodynamics and is crucial for understanding phase changes and energy transformations. By understanding the methods and factors involved in measuring the latent heat of fusion of ice, science students can gain valuable insights into the behavior of matter and the principles of energy conservation.
References:
- Measuring the Latent Heat of Fusion of Ice
- Measuring the Heat of Fusion of Ice
- Latent Heat of Fusion of Ice Experiment
- Latent Heat of Fusion of Ice Experiment (Video)
- Latent Heat of Fusion of Ice Experiment (PDF)

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.