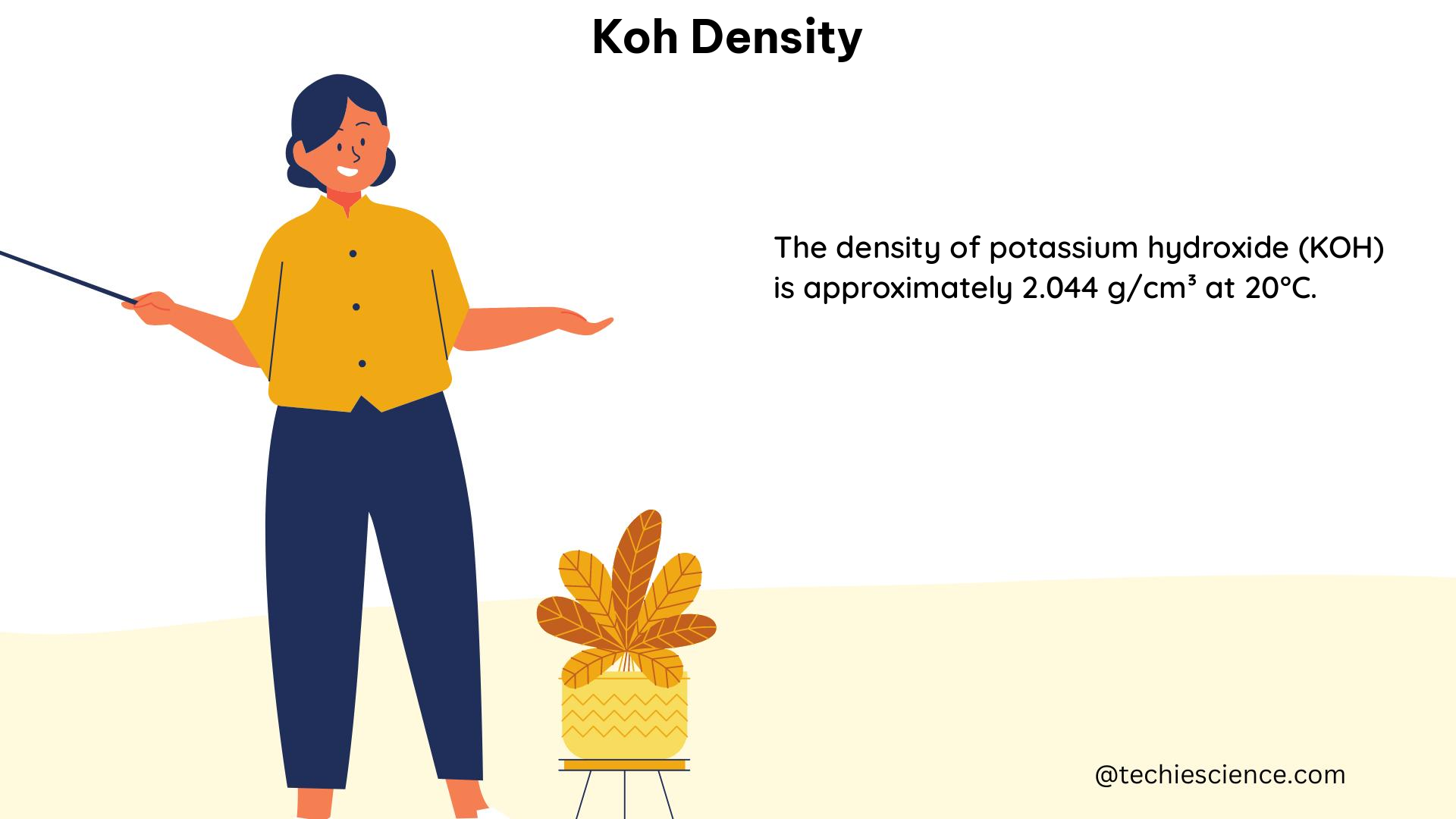
Potassium hydroxide (KOH) is a widely used chemical compound with numerous industrial and scientific applications. The density of KOH solutions is a crucial physical property that has significant implications for various processes and calculations. This comprehensive guide delves into the theoretical foundations, empirical correlations, experimental data, and practical implications of KOH density, providing a valuable resource for physics students and professionals working with KOH solutions.
Theoretical Background
The density of a substance is defined as the mass per unit volume, typically expressed in grams per cubic centimeter (g/cm³) or kilograms per cubic meter (kg/m³). In the context of KOH solutions, the density is a function of several variables, including the concentration of KOH, temperature, and the presence of other solutes, such as water.
The relationship between these variables can be expressed through the following general equation:
ρ = f(C, T, S)
Where:
– ρ is the density of the KOH solution
– C is the concentration of KOH (in wt% or mol/L)
– T is the temperature of the solution (in °C or K)
– S represents the presence and concentration of other solutes
This relationship is complex and can be described through empirical correlations derived from experimental data.
Empirical Correlations
To estimate the density of KOH solutions, researchers have developed various empirical correlations based on experimental data. One widely used correlation is the following equation:
ρ(T, wt%) = A + BT + C(wt%) + DT^2 + E(wt%)^2 + FT(wt%) + G(wt%)^2T
Where:
– ρ is the density of the KOH solution (in g/cm³ or kg/m³)
– T is the temperature of the solution (in °C)
– wt% is the concentration of KOH (in weight percent)
– A, B, C, D, E, F, and G are empirical coefficients that depend on the specific range of temperatures and concentrations
The values of these coefficients can be determined by fitting the equation to experimental data. For example, the following set of coefficients can be used for KOH solutions with concentrations up to 50 wt% and temperatures ranging from -53.8°C to 98.9°C:
| Coefficient | Value |
|---|---|
| A | 1.0479 |
| B | -0.0008 |
| C | 0.0058 |
| D | 0.0000 |
| E | 0.0000 |
| F | -0.0001 |
| G | 0.0000 |
These empirical correlations provide a convenient way to estimate the density of KOH solutions based on the concentration and temperature, without the need for extensive experimental measurements.
Experimental Data
Numerous studies have been conducted to measure the density of KOH solutions at various concentrations and temperatures. Some of the key experimental data sources include:
- Klochko, V.A., and Godneva, N.B., “Specific conductivity of aqueous solutions of alkali hydroxides at different temperatures,” Journal of Applied Chemistry of Ukraine, vol. 67, no. 1, pp. 38-45, 2006.
- Seidel, R., et al., “Anisotropic Etching of Crystalline Silicon in Alkaline Solutions,” Journal of the Electrochemical Society, vol. 137, no. 11, pp. 3612-3626, 2000.
- “Calculation method for potassium hydroxide aqueous solution,” Saitama University, 2011.
- “Determining the density and vapor pressure of KOH solutions as required,” NASA Technical Reports Server, 1966.
These sources provide comprehensive data on the density of KOH solutions, covering a wide range of concentrations and temperatures. For example, the NASA Technical Reports Server data includes density measurements for KOH solutions at temperatures ranging from -53.8°C to 98.9°C and concentrations up to 50 wt%.
The experimental data consistently shows that the density of KOH solutions decreases with increasing temperature and concentration. This behavior can be attributed to the thermal expansion of the solution and the dilution effect caused by the addition of water or other solutes.
Practical Implications
The density of KOH solutions has several practical implications in various applications, including:
-
Preparation of KOH Solutions: The density of KOH solutions can be used to determine the appropriate amount of KOH and water required to prepare a solution with a desired concentration.
-
Measurement of KOH Concentration: The density of KOH solutions can be used to indirectly measure the concentration of KOH in a solution, as demonstrated in the US8628678B2 patent.
-
Design of Chemical Processes: The density of KOH solutions can affect the performance of chemical processes, such as the etching of silicon in alkaline solutions, as discussed in the Seidel et al. study.
-
Electrochemical Applications: The density of KOH solutions is a crucial parameter in electrochemical applications, such as fuel cells and batteries, where it can influence the transport of ions and the overall performance of the system.
-
Environmental and Safety Considerations: The density of KOH solutions is an important factor in the handling, storage, and transportation of these chemicals, as it can affect the volume and weight of the solutions, which are relevant for safety and environmental regulations.
By understanding the theoretical foundations, empirical correlations, and experimental data related to KOH density, physics students and professionals can make informed decisions and perform accurate calculations in a wide range of applications involving KOH solutions.
References
- Klochko, V.A., and Godneva, N.B., “Specific conductivity of aqueous solutions of alkali hydroxides at different temperatures,” Journal of Applied Chemistry of Ukraine, vol. 67, no. 1, pp. 38-45, 2006.
- Seidel, R., et al., “Anisotropic Etching of Crystalline Silicon in Alkaline Solutions,” Journal of the Electrochemical Society, vol. 137, no. 11, pp. 3612-3626, 2000.
- “Calculation method for potassium hydroxide aqueous solution,” Saitama University, 2011.
- US8628678B2 – Method for measuring the active KOH concentration, Google Patents, 2014.
- “Determining the density and vapor pressure of KOH solutions as required,” NASA Technical Reports Server, 1966.

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.