KNO2 or Potassium nitrite is an inorganic compound of K+ and NO2–. Let us discuss some facts related to KNO2 in detail.
KNO2 or Potassium nitrite is hygroscopic substance with molar mass 85.10379 g/mol. It is deliquescent and its density is 1.914 g/cm3. Its melting point and boiling point is 440.020 C and 5370 C respectively. It is found to be a good oxidising agent and is very toxic too.
Sometimes potassium nitrite can be mutagenic in nature. Let us discuss the structure, formal charge, angle, hybridisation and many other facts related to KNO2 in this article.
How to draw KNO2 Structure?
Crystal structure of a compound is obtained when atoms or group of atoms joined together. Let us look into the structure of KNO2.
- The crystal structure of KNO2 is found through oscillating single crystal photographs.
- The results were spherical shaped granule crystals without face and was anhydrous.
- Its basic crystal structure is monoclinic with a,b,c values are 4.45 A0, 4.99 A0, 7.31 A0.
- The observed density is 1.915 g per cc and the number of molecules in the unit cell is 2.01.
- The rotation and laue photographs leads to the following monoclinic crystal structure of KNO2.

KNO2 Structure Shape
The shape of a molecule can be predicted by knowing its hybridization. Let us look into the shape of KNO2.
The shape of KNO2 structure is bent. KNO2 is an ionic compound and its shape is determined by the shape of nitrite. Nitrite is bent molecule or v shaped molecule because nitrogen and oxygen both contain lone pairs.
KNO2 Structure Formal Charge
Formal charge is a hypothetical charge given to an atom and it can be any integers. Let us calculate the formal charge of KNO2.
The formal charge of KNO2 is 0 for potassium and oxygen. Formal charge is 1 for nitrogen. The formal charge finding equation is
Formal charge= valence electrons — non bonding electrons — no. of bonds
- Formal charge of oxygen = 0
- Formal charge of potassium=0
- Formal charge of nitrogen = 1
KNO2 Structure Angle
Angle can be distorted for certain molecules having lone pairs in the central atom. Let us look into the bond angle of KNO2.
The bond angle of KNO2 structure is 1200 due to the presence of lone pairs in nitrogen.
KNO2 Structure Lone Pairs
Lone pairs or non bonding electrons are actually one thing. Let us know about the lone pairs in KNO2.
The lone pairs in KNO2 is 6. One oxygen has two, one has three, and nitrogen has one pair. On combining there are 6 lone pairs or 12 non bonding electrons found in potassium nitrite.
KNO2 Valence Electrons
Valence Electrons are usually transferred between the atoms during chemical reactions. Let us research KNO2 valence electrons.
There are 18 valence electrons in KNO2 with one, nitrogen with 5 and oxygen with 6 .
KNO2 Hybridization
Hybridisation is the formation of new orbitals as a result overlapping of atomic orbitals. Let us see the hybridisation in KNO2.
The hybridisation of nitrite in KNO2 is sp2 . One 2s and two 2p orbitals of nitrogen overlaps to for sp2 hybrid orbitals. The oxygen’s partially filled 2p orbitals share electrons with the sp2 orbitals.
The unhybridised p orbital of oxygen overlaps with 2p orbital of nitrogen to make pi bond.
KNO2 Solubility
Ionic compounds tend not to dissolve in non polar solvents. Let us examine KNO2 solubility.
KNO2 has been proven to be water soluble. It gets highly get dissolved in water and can’t even separate easily.KNO2 is soluble in water because it is polar in nature.
KNO2 is soluble in the following solvents
- Ethanol
- Ammonia
Is KNO2 Solid or Liquid?
Solids and liquids have definite volume and density but gas doesn’t have any volume. Let us see KNO2 is solid or liquid.
KNO2 is a solid substance. It is a crystalline substance which either white or yellow in color. It is powdered in nature.
Is KNO2 Polar or Non Polar?
Polar substance has charged ions in it and has a definite value of dipole moment. Let us see whether KNO2 is polar or not.
KNO2 is a polar substance. K+ and NO2– ions comprises together to form KNO2 which is polar. The electronegativity of potassium, nitrogen and oxygen is 0.82,3.04 and 3.44 .
Since there is huge difference in their electronegativity values and they are charged molecules they are polar compounds.
Is KNO2 Acidic, Basic or Salt?
Acids and bases combines together to give the neutral substance called salt as a result of neutralisation reaction. Let us check about the acidity, basicity and salt like nature of KNO2.
Strong bases and weak acids combine to generate the salt known as KNO2.KNO2 is formed when KOH a strong base reacts with HNO2 a weak acid.
Here a strong base and weak acid is combined so the salt formed called KNO2 is a basic salt with pH value is 8.40.
Is KNO2 Electrolyte?
An electrolyte can conduct electricity easily in solution. Let us know do KNO2 acts as an electrolyte or not.
KNO2 is an electrolyte because its an ionic compound. It can dissociate well in water to give its corresponding ions from its lattice site. These ions can easily conduct electricity.
Is KNO2 Ionic or Covalent?
Ionic and covalent compounds are two classes of compounds . Let us see KNO2 is ionic or covalent.
KNO2 is an ionic compound. Potassium donates one electron to NO2 to form the cation K+and anion NO2– respectively. These ions are joined by the simple electrostatic force of attraction to form the compound.
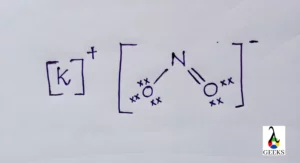
Conclusion
KNO2 or potassium nitrite is a inorganic salt used for manufacturing of heat transfer cells, as a food additive and preservative. It is stored away from combustible materials, acids and reducing agents in cool, dry ventilated locations.

Hi… I am Aparna Dev, a chemistry Postgraduate with a good understanding of chemistry concepts. I am working in Kerala Minerals and Metals Limited Kollam with experience in the development of electrocatalysts as a part of post graduate thesis.
Let’s connect through LinkedIn-https://www.linkedin.com/in/aparna-dev-76a8751b9