Kerosene density is a crucial physical property that can be used to determine the identity and quality of kerosene. This comprehensive guide will provide you with advanced details and technical specifications, theoretical explanations, hands-on instructions, and numerical problems to help you understand and measure the density of kerosene accurately.
Advanced Details and Technical Specifications
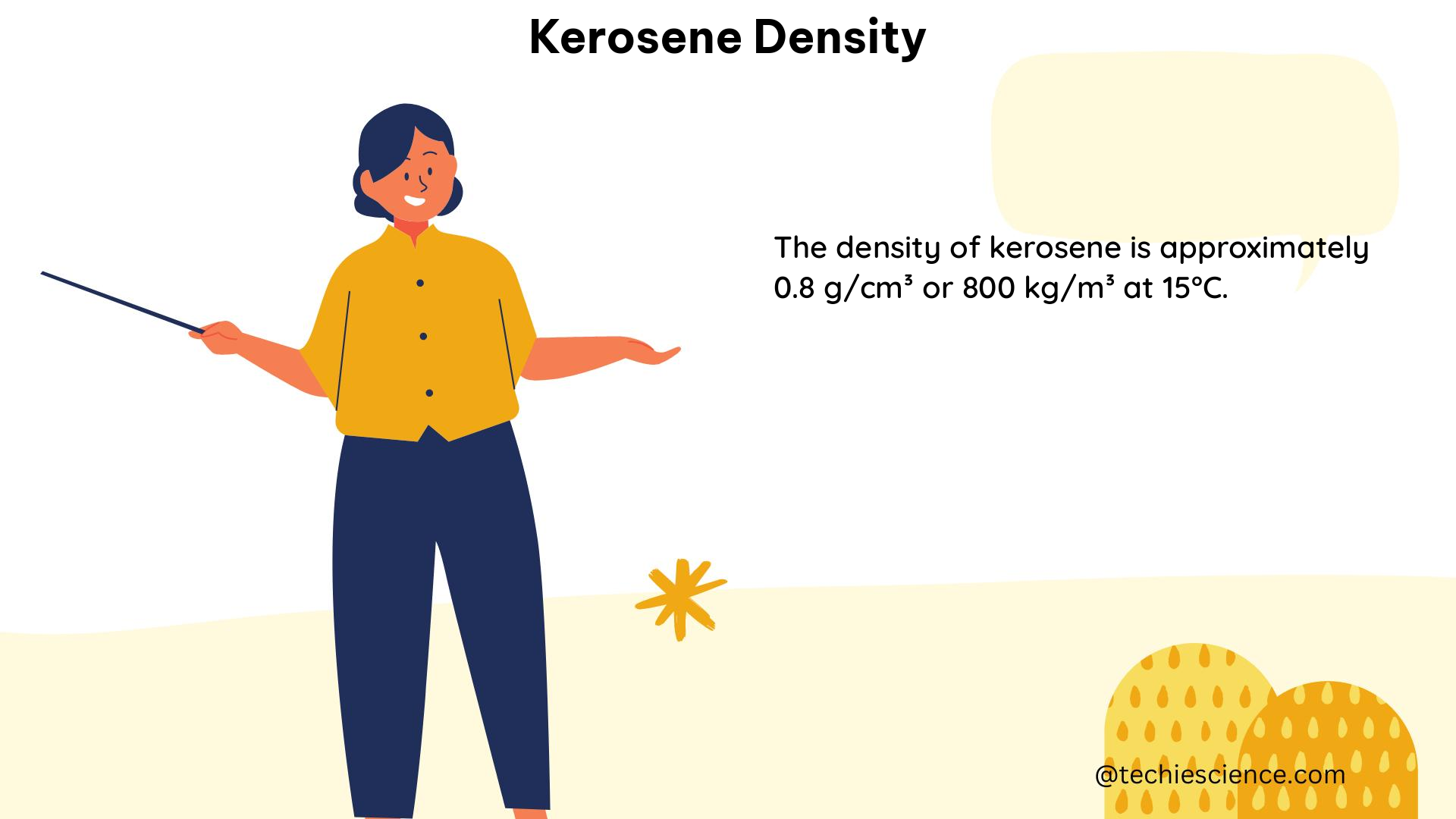
The density of kerosene is typically measured in units of g/cm³ or g/mL for liquids. According to the regulatory specifications for automotive diesel fuel, the density of diesel fuel should remain within 820-845 kg/m³ at 15 °C. However, the addition of kerosene lowers the density of diesel fuel. In a study, it was found that the density of diesel fuel decreased gradually from 830 kg/m³ to 821 kg/m³ with the final addition of 40% kerosene (v/v). The density of PDS kerosene, which is used as an adulterant, was measured to be 802 kg/m³.
Theoretical Explanation
Density is a physical property that describes the mass of a substance per unit volume. It is an intensive property, meaning that it does not depend on the amount of substance present. The density of a substance can be used to determine its identity and quality. For example, the density of kerosene is typically higher than the density of diesel fuel, and the addition of kerosene to diesel fuel can lower its density.
In the regulatory specifications for automotive diesel fuel, the density of diesel fuel should remain within 820-845 kg/m³ at 15 °C. This range is based on the properties of pure diesel fuel and is used to ensure that the fuel meets certain performance and safety standards. However, the addition of kerosene or other adulterants can lower the density of diesel fuel and potentially affect its performance and safety.
Hands-on Details
To measure the density of kerosene, follow these steps:
- Obtain a known quantity of kerosene, such as 100 mL.
- Measure the mass of the kerosene using an analytical balance. Record the mass to the correct number of significant figures and with the proper units.
- Measure the volume of the kerosene using a volumetric pipet or graduated cylinder. Record the volume to the correct number of significant figures and with the proper units.
- Calculate the density by dividing the mass by the volume. Record the density to the correct number of significant figures and with the proper units.
- To minimize random errors, repeat the measurements several times and calculate the mean density by adding together the values for all trials and dividing by the number of trials.
Numerical Problems
- A sample of kerosene has a mass of 125.6 g and a volume of 100.0 mL. Calculate the density of the kerosene.
Answer: The density of the kerosene is 1.256 g/mL.
- A sample of diesel fuel has a density of 830 kg/m³ at 15 °C. If 10% kerosene (v/v) is added to the diesel fuel, what is the new density of the mixture?
Answer: The new density of the mixture is 821 kg/m³.
Figures and Data Points
- Density of kerosene: 802 kg/m³
- Density of diesel fuel: 830 kg/m³ at 15 °C
- Kinematic viscosity of kerosene: 1.3-4.1 cSt at 40 °C
Unique Perspective
While density measurements can indicate adulterations if the levels of adulterants are significantly high, they cannot conclusively determine the presence of kerosene in diesel fuel. Other physical properties such as kinematic viscosity, flash point, and distillation range can also be used to determine the identity and quality of kerosene. These properties can provide valuable information about the composition and purity of kerosene and can help ensure that it meets certain performance and safety standards.
In addition to its use as a fuel, kerosene has many other applications, such as in lighting, heating, and cooking. It is also used as a solvent and a cleaning agent. Understanding the physical properties of kerosene, including its density, can help ensure that it is used safely and effectively in these applications.
Reference Links:
- Measurements and Calculations – SharpSchool: https://cdnsm5-ss6.sharpschool.com/UserFiles/Servers/Server_7985/File/Mr.%20Novak%27s%20Chemistry/CH%202%20-%20STUDY%20GUIDE%20ANSWER%20KEY_study_gd_ak.pdf
- Experiment 1: Mass, Volume, and Density – Science Department: https://science.valenciacollege.edu/chemistry/experiments/1045-exp1-massvolumedensity.pdf
- Determination of kerosene as an adulterant in diesel through physicochemical analysis: https://link.springer.com/article/10.1007/s42452-019-0637-7

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.