Diamond is a unique material that exhibits both insulating and semiconducting properties, depending on various factors. This comprehensive guide will delve into the technical details and specific characteristics that make diamond an exceptional insulator, as well as the factors that can influence its electrical conductivity.
Understanding Diamond’s Bandgap
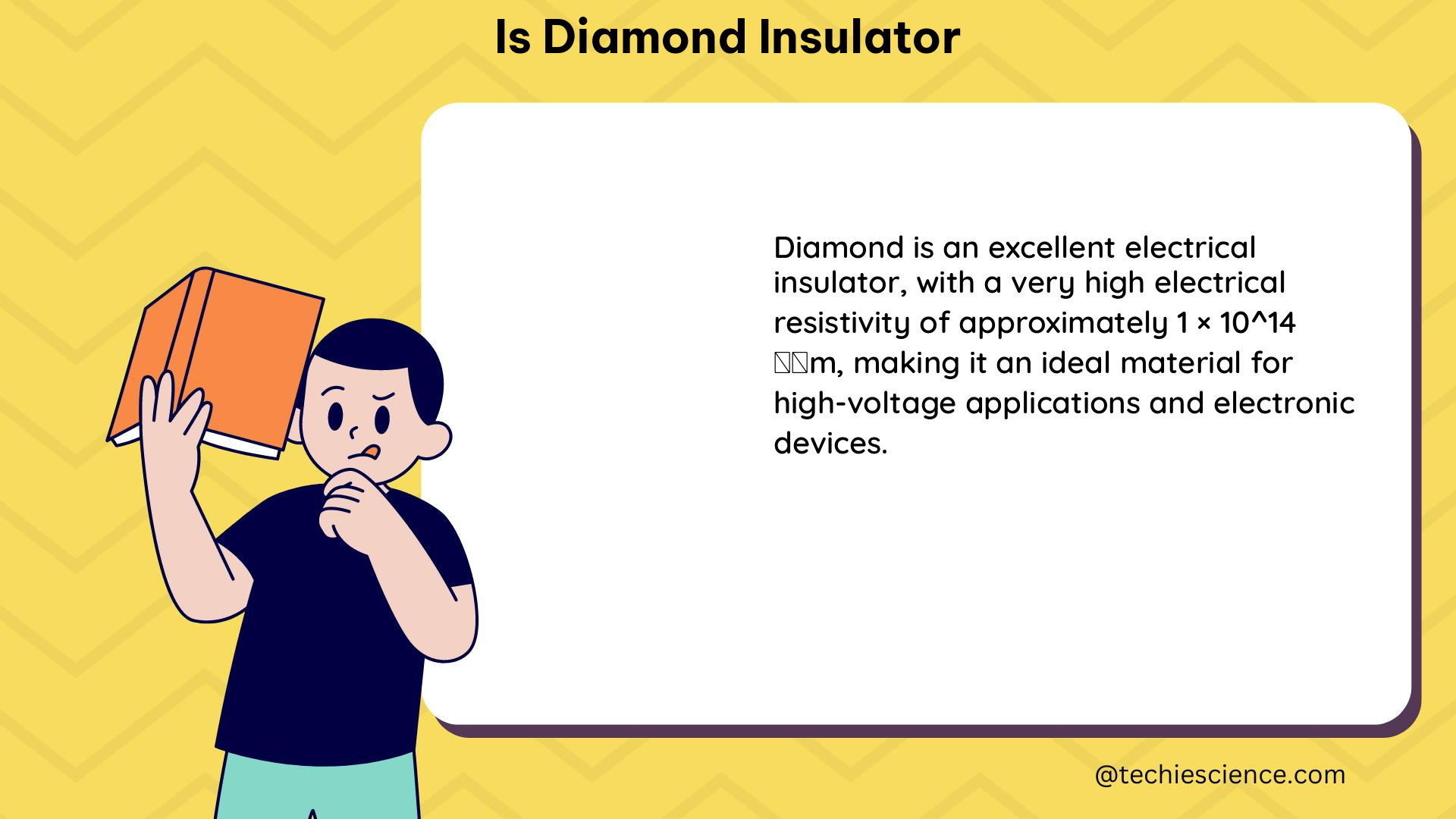
The primary reason why diamond is considered an insulator is its wide bandgap, which is the energy difference between the valence band (VBM) and the conduction band (CBM). The bandgap of diamond is approximately 5.5 eV, which is significantly larger than the bandgap of typical semiconductors like silicon (1.1 eV).
This wide bandgap makes it extremely difficult for electrons to be excited from the valence band to the conduction band, resulting in very low electrical conductivity. The energy required to overcome this bandgap is much higher than the thermal energy available at room temperature, effectively trapping the electrons in the valence band.
The wide bandgap of diamond can be expressed mathematically as:
E_g = E_c - E_v
Where:
– E_g is the bandgap energy
– E_c is the energy of the conduction band
– E_v is the energy of the valence band
For diamond, the typical value of E_g is around 5.5 eV.
Impurities and Defects in Diamond
While diamond is primarily an insulator, its electrical conductivity can be influenced by the presence of impurities and defects within the crystal structure. These imperfections can introduce additional energy levels within the bandgap, altering the material’s electronic properties.
Boron Impurities
One of the most notable examples is the introduction of boron impurities in the diamond lattice. Boron atoms can substitute for carbon atoms, creating electron holes in the valence band. These holes can increase the electrical conductivity of the diamond, allowing it to behave as a semiconductor.
This phenomenon is observed in some blue diamonds, where the boron impurities give rise to a characteristic blue color. The presence of these boron-related defects can significantly enhance the electrical conductivity of the diamond.
Hydrogen-related Species
Another factor that can influence the electrical conductivity of diamond is the presence of hydrogen-related species adsorbed on the surface. In nominally undoped diamond grown by chemical vapor deposition (CVD), the adsorption of these hydrogen-related species can lead to substantial conductivity.
However, this surface-induced conductivity can be removed by annealing or other surface treatments, as the hydrogen-related species are desorbed from the surface.
Mechanical Deformation
Interestingly, thin needles of diamond can have their electronic bandgap varied from the normal 5.6 eV to near zero by selective mechanical deformation. This selective deformation can alter the crystal structure and electronic properties of the diamond, effectively transforming it from an insulator to a material with a near-zero bandgap.
Thermal Properties of Diamond
In addition to its electrical properties, diamond is also an excellent thermal insulator due to its unique physical characteristics.
Thermal Conductivity
Diamond has an exceptionally high thermal conductivity, approximately 2000 W/(m·K), which is about five times that of copper and twenty times that of silicon. This high thermal conductivity is a result of the strong covalent bonds and the efficient transfer of phonons (lattice vibrations) within the diamond crystal structure.
Thermal Expansion Coefficient
Diamond also has a very low thermal expansion coefficient, which means that it undergoes minimal dimensional changes with changes in temperature. This low thermal expansion coefficient contributes to diamond’s excellent thermal insulating properties, as it minimizes the risk of thermal-induced stresses and deformations.
The combination of high thermal conductivity and low thermal expansion coefficient makes diamond an ideal material for thermal management applications, where efficient heat dissipation and dimensional stability are crucial.
Applications of Diamond as an Insulator
Given its unique insulating and thermal properties, diamond finds various applications in the field of electronics and thermal management:
-
Semiconductor Devices: The wide bandgap and insulating properties of diamond make it a suitable material for high-power, high-frequency, and high-temperature semiconductor devices, such as power electronics and radio frequency (RF) devices.
-
Thermal Management: The exceptional thermal conductivity of diamond makes it an excellent material for heat sinks, heat spreaders, and other thermal management components in electronic devices and systems.
-
Optical Windows: The transparency of diamond in the visible and infrared regions of the electromagnetic spectrum, combined with its high thermal conductivity, makes it an ideal material for optical windows in high-power laser applications and harsh environments.
-
Cutting Tools: The hardness and wear resistance of diamond make it a valuable material for cutting tools, where its insulating properties help minimize electrical discharge and improve tool life.
-
Jewelry and Gemstones: The unique optical and physical properties of diamond, such as its high refractive index and ability to withstand high temperatures, make it a highly sought-after material for jewelry and gemstone applications.
Conclusion
In summary, diamond is primarily considered an insulator due to its wide bandgap, which makes it difficult for electrons to be excited from the valence band to the conduction band. However, the electrical conductivity of diamond can be influenced by the presence of impurities and defects, such as boron impurities and hydrogen-related species, as well as by selective mechanical deformation.
Additionally, diamond’s exceptional thermal properties, including high thermal conductivity and low thermal expansion coefficient, make it an excellent material for various applications in electronics, thermal management, and beyond.
References:
- Diamond – Book chapter – IOPscience, https://iopscience.iop.org/book/mono/978-1-64327-338-9/chapter/bk978-1-64327-338-9ch7
- Imperfections in natural diamond: the key to understanding diamond, https://link.springer.com/article/10.1007/s40766-023-00045-6
- Diamond – Wikipedia, https://en.wikipedia.org/wiki/Diamond
- Absolute energy levels in nanodiamonds of different origins, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10448352/
- Is silicon a semiconductor or insulator? – Physics Stack Exchange, https://physics.stackexchange.com/questions/644832/is-silicon-a-semiconductor-or-insulator
Hi, I’m Akshita Mapari. I have done M.Sc. in Physics. I have worked on projects like Numerical modeling of winds and waves during cyclone, Physics of toys and mechanized thrill machines in amusement park based on Classical Mechanics. I have pursued a course on Arduino and have accomplished some mini projects on Arduino UNO. I always like to explore new zones in the field of science. I personally believe that learning is more enthusiastic when learnt with creativity. Apart from this, I like to read, travel, strumming on guitar, identifying rocks and strata, photography and playing chess.