Iodine Trichloride is an inorganic compound made up of iodine and chlorine and is known as an interhalogen inorganic compound. Let us discuss the properties of Iodine Trichloride.
Iodine trichloride can also convert into an organic compound by eliminating the halogen group from it and can do many organic syntheses further and is highly corrosive in nature for human tissue and highly toxic for ingestion.
In this article, we will discuss the chemical formula, molar mass, color, oxidation state, and viscosity with a detailed explanation.
Iodine trichloride IUPAC name
The IUPAC name (international union of pure and applied chemistry) of Iodine trichloride is Iodine trichloride itself.
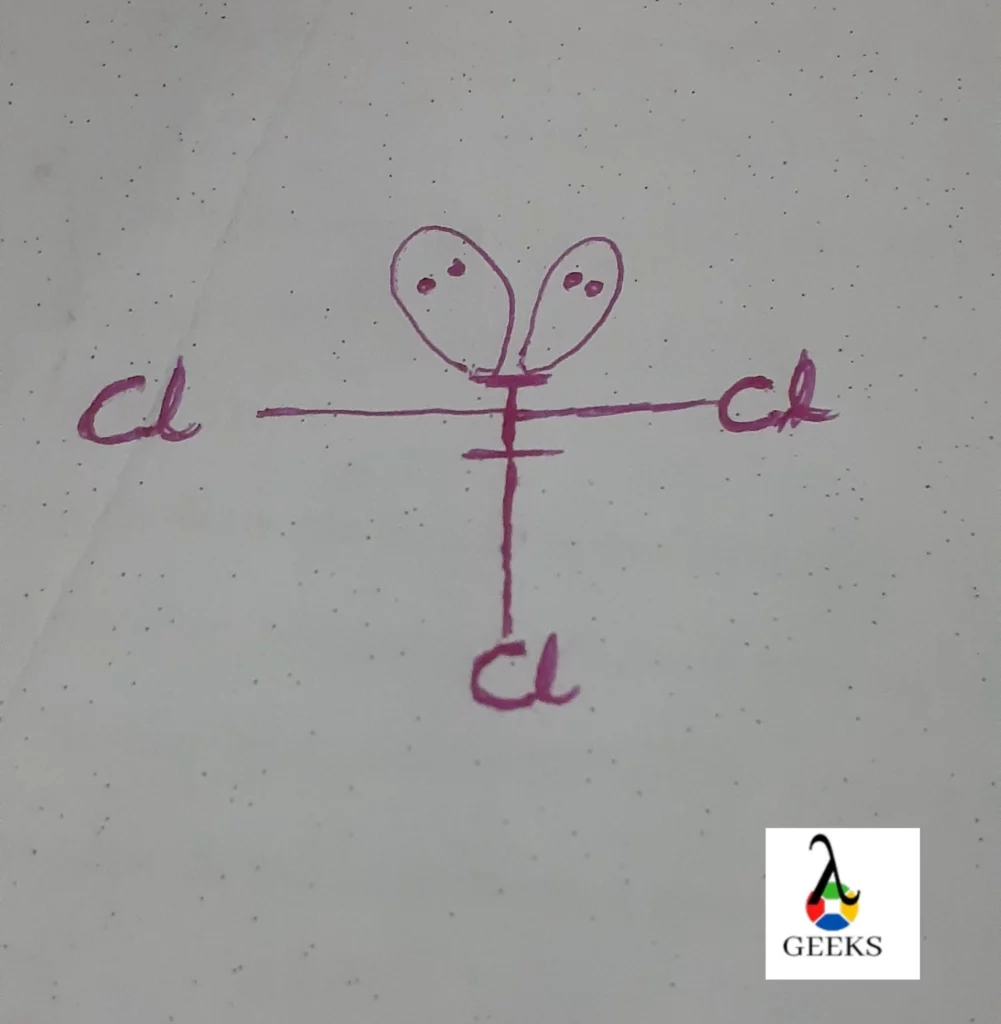
Iodine trichloride chemical formula
Iodine trichloride has the chemical formula ICl3 . In this chemical formula, the Iodine atoms are bounded by 3 Chlorine atoms as iodine have 7 electrons in the outer shell it makes 3 bonds with chlorine, and the remaining 4 electrons will be as 2 lone pair on iodine.
Iodine trichloride CAS number
The CAS registry number of Iodine trichloride is 865-44-1 which is an authentic numeric identifier that contains up to 10 digits.
Iodine trichloride ChemSpider ID
Iodine trichloride has the ChemSpider ID is a free chemical structure database 63265.
Iodine trichloride chemical classification
- Iodine trichloride is an interhalogen chemical compound because both of them belong to the halogen group of the periodic table.
- Iodine trichloride is a highly reactive chemical compound because of the highly unstable central atom which is Iodine.
Iodine trichloride molar mass
The molar mass of Iodine trichloride is 466.53 g/mol.
Iodine trichloride color
Pure Iodine trichloride is bright yellow in color but if kept in a closed black bottle if exposed to the air the color turns red because of the presence of Iodine in the chemical compound because iodine is unstable and reactive.
Iodine trichloride viscosity
Iodine trichloride is a solid powdered or crystal compound and not viscous because Iodine chloride is not a liquid compound and viscosity is contained by only liquid chemical compounds.
Iodine trichloride molar density
The molar density of Iodine trichloride is 3111 kg/m³ at 15°C as it has a density of 3.11 g/cm³.
Iodine trichloride melting point
The melting point of Iodine trichloride is 63°C or 145.40°F.
Iodine trichloride boiling point
The boiling point of Iodine trichloride is 105°C.
Iodine trichloride state at room temperature
Iodine trichloride is in a solid state at room temperature or in a shining crystal solid form and in molecular lattice form too.
Iodine trichloride covalent bond
Pure Iodine trichloride contains covalent bonds. These bonds become polar covalent bonds because of the difference in the electronegativity of chlorine and iodine electron pair shifts to chlorine and iodine attends positive charge and the bond becomes a polar covalent bond.
Iodine trichloride covalent radius
The covalent radius of Iodine trichloride is 139 pm.
Iodine trichloride electron configurations
The electronic configuration is the division of the electrons in the atomic orbitals. Let us explain the electronic configuration of iodine trichloride.
Electronic configuration of Iodine 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p5 and chlorine 1s2 2s2 2p6 3s2 3p5.
Iodine trichloride oxidation state
Oxidation state of the Iodine chloride is +3 because the central atom donates the 3 electrons to the 3 chlorine atoms and attends the +3 charge which is the oxidation state.
Iodine trichloride acidity/alkaline
Pure Iodine chloride does not contain acidity or alkalinity because of the lack of hydrogen and hydronium ions which is responsible for acidity or alkalinity.
Is iodine trichloride odourless
Iodine trichloride is an odourless chemical compound and does not produce any odour while heating too.
Is iodine trichloride paramagnetic
Paramagnetism is a phenomenon in which a single electron is left in the last shell. Let us explain the paramagnetic Iodine trichloride.
Iodine Trichloride is paramagnetic because there are single electrons in multiple orbitals left and they show colors too.
Iodine trichloride hydrates
Iodine trichloride does not produce any hydrides but reacts with water under vigorous or rare conditions.
5 ICl3 + 9 H2O → 15 HCl + 3 HIO3
Iodine trichloride crystal structure
Iodine trichloride has a crystal triclinic shape known as triclinic crystals they are solid and very compact and unbreakable too.
Iodine trichloride polarity and conductivity
- Iodine trichloride shows polarity because of the difference in the electronegativity of iodine and chlorine.
- Iodine chloride shows conductivity only in a molten state, after dissociation not in a solid state.
Iodine trichloride reaction with acid
Iodine chloride does not react with acid because of the absence of hydrogen ions.
Iodine trichloride reaction with base
Iodine trichloride does not react with the base as it is an analytical agent but iodine only can react with the base.
3 I2(s) + 6 OH−(aq) —–> IO3−(aq) + 5 I−(aq) + 3 H2O(l)
Iodine trichloride reaction with oxide
Iodine trichloride does not react with oxide but works as a good oxidizing agent.
Iodine trichloride reaction with metal
Iodine trichloride does not react with metal because of the presence of lone pair of electrons in a central atom.
Conclusion
Iodine trichloride is a highly toxic and reactive compound and easily catches fire used for analyzing organic compounds mostly in the test reaction of iodoform and works as a topical antiseptic too.

Hi….I am Soumya Chourasia, completed my Master’s in Chemistry. I am working as a Subject Matter Expert in Chemistry. Before this, I used to teach chemistry for competitive examinations and school as well.
Let’s connect through LinkedIn here,