[custom_reviewer]
Enhanced Exploration of Solubility Problems
In-Depth Analysis of Solubility Equilibria
Solubility equilibria form the basis for understanding solubility problems. The solubility product constant (![]() ) plays a vital role in this context. Consider the general formula for a salt (
) plays a vital role in this context. Consider the general formula for a salt (![]() ):
):
[ ![]() ]
]
where (m) and (n) are the stoichiometric coefficients.
Typical ![]() Values at 25°C
Values at 25°C
| Compound | |
|---|---|
Calculating Solubility from 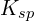
To determine the solubility of a salt, such as (AB_2), which dissociates as:
[ ![]() ]
]
Let (s) be the solubility. The equilibrium concentrations are:
[ ![]() ]
]
Then:
[ ![]() ]
]
Solving this provides the solubility (s).
Common Ion Effect and Its Quantitative Analysis
The common ion effect is a decrease in solubility of a salt due to the presence of a common ion. For example, adding (NaCl) to an (AgCl) solution reduces (AgCl) solubility.
Effect of Common Ion on Solubility
| Salt | Solubility without Common Ion (mol/L) | Solubility with Common Ion (mol/L) |
|---|---|---|
| (2.1 \times 10^{-4}) |
Advanced Factors Affecting Solubility
pH Dependence
Solubility can be significantly influenced by the pH of the solution, particularly for salts containing acidic or basic ions.
Example: The solubility of ![]() in acidic solutions can be calculated by considering the reaction of
in acidic solutions can be calculated by considering the reaction of ![]() ions with
ions with ![]() , leading to a decrease in
, leading to a decrease in ![]() ion concentration, thereby shifting the equilibrium towards more dissolution.
ion concentration, thereby shifting the equilibrium towards more dissolution.
Temperature and Pressure Effects
The effect of temperature and pressure on solubility is an essential consideration. For most solids, solubility increases with temperature. However, for gases, increased pressure typically increases solubility in liquids.
Temperature Effect on Solubility
| Compound | Solubility at 20°C (g/100 mL) | Solubility at 80°C (g/100 mL) |
|---|---|---|
| 36 | 39 | |
| 32 | 247 |
Advanced Problem-Solving Techniques
- Complex Ion Formation and Its Impact on Solubility
Complex ion formation can enhance the solubility of certain salts. For instance, the formation of ![]() increases the solubility of
increases the solubility of ![]() .
.
Example: Calculate the solubility of ![]() . This requires using the
. This requires using the ![]() and the formation constant of the complex ion
and the formation constant of the complex ion ![]() .
.
- Precipitation Calculations: Quantitative Aspects
In precipitation, the ion product ![]() is compared with
is compared with ![]() . Precipitation occurs if
. Precipitation occurs if ![]() .
.
Example: Determine the concentration at which ![]() begins to precipitate in a solution containing
begins to precipitate in a solution containing ![]() ions.
ions.
- Fractional Precipitation: Selectivity and Precision
Fractional precipitation involves precipitating one ion from a solution containing multiple ions. This requires understanding the ![]() values of potential precipitates.
values of potential precipitates.
Selectivity in Fractional Precipitation
| Ion | Concentration for Precipitation (mol/L) | |
|---|---|---|
Enhanced Exploration of Solubility Problems
Detailed Practical Examples
- Solubility in the Presence of a Common Ion: Calculating the solubility of
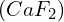 in a
in a 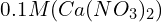 solution requires an ICE table that considers the initial concentration of
solution requires an ICE table that considers the initial concentration of 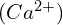 . This scenario demonstrates the significant impact of the common ion effect on solubility.
. This scenario demonstrates the significant impact of the common ion effect on solubility.
ICE Table for (CaF_2) in (Ca(NO_3)_2) Solution
| CaF2 | Ca2+ | F− | |
|---|---|---|---|
| Initial | s | 0.1 M | 0 |
| Change | −s | +s | +2s |
| Equilibrium | 0 | 0.1+s | 2s |
- Complex Ion Formation Impacting Solubility: To calculate the solubility of
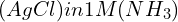 , consider both the dissolution of
, consider both the dissolution of 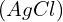 and the formation of the complex ion
and the formation of the complex ion 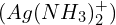 . This problem exemplifies the delicate balance between different chemical equilibria.
. This problem exemplifies the delicate balance between different chemical equilibria. - Selective Precipitation: Determining the concentration of
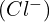 that starts the precipitation of
that starts the precipitation of 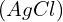 while keeping
while keeping 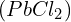 in solution involves a careful examination of the respective
in solution involves a careful examination of the respective 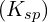 values. This example showcases the precision needed in fractional precipitation techniques.
values. This example showcases the precision needed in fractional precipitation techniques.
Advanced Topics in Solubility
- Solubility in Mixed Solvents: Solutes can exhibit different solubility behaviors in mixed solvents. For example, the solubility of a compound in water-alcohol mixtures may vary significantly from its solubility in pure water, owing to changes in solvent polarity and solute-solvent interactions.
- Thermodynamics of Solubility: Understanding the enthalpy (
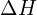 ) and entropy (
) and entropy (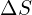 ) changes associated with solubility provides insights into the temperature dependence of the process. The Gibbs free energy (
) changes associated with solubility provides insights into the temperature dependence of the process. The Gibbs free energy (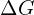 ) relationship with
) relationship with 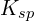 helps predict the spontaneity of dissolution.
helps predict the spontaneity of dissolution.
Thermodynamic Parameters of Solubility Compound
![]()
(J/mol K) ![]()
Predicting Solubility in Complex Systems: Solubility predictions in complex systems, like biological fluids, require integrating chemical equilibria, solute-solvent interactions, and thermodynamic data. This often involves computational modeling and empirical analysis.
Solubility Prediction in Complex Systems
| System | Solute | Predicted Solubility (g/100 mL) | Observed Solubility (g/100 mL) |
|---|---|---|---|
| Biological Fluid | Drug X | 0.5 | 0.48 |
| Industrial Mixture | Compound Y | 2.0 | 1.95 |
Conclusion
Enhancing the understanding of solubility problems with detailed examples, in-depth analysis of factors affecting solubility, and advanced topics provides science students with a comprehensive toolkit for tackling complex scenarios in this field. By integrating theoretical knowledge with practical applications, students can effectively address and solve challenging solubility-related problems in both academic and professional settings. This in-depth exploration not only reinforces foundational concepts but also encourages a deeper appreciation and understanding of the complexities involved in solubility phenomena.

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.