Summary
Finding the mass of an object is a fundamental concept in physics, and it is essential for understanding various physical phenomena. This comprehensive guide will provide you with a detailed understanding of the different methods and techniques used to determine the mass of an object, including the use of balances, density calculations, and advanced mass measurement techniques. Whether you are a beginner or an experienced physics student, this guide will equip you with the knowledge and tools necessary to accurately measure the mass of any object.
Understanding Mass and Weight
Mass is a fundamental property of an object that represents the amount of matter it contains. It is a scalar quantity, meaning it has a magnitude but no direction. The mass of an object remains constant regardless of its location or the forces acting upon it.
On the other hand, weight is the force exerted on an object due to the acceleration of gravity. Weight is a vector quantity, meaning it has both magnitude and direction. The weight of an object can change depending on the gravitational force acting on it, which can vary depending on the object’s location.
The relationship between mass and weight can be expressed using the following equation:
Weight = Mass × Acceleration due to gravity
It is important to note that while mass and weight are related, they are not the same. Mass is an intrinsic property of an object, while weight is a measure of the force acting on the object due to gravity.
Measuring Mass Using Balances
The most common method for measuring the mass of an object is by using a balance. Balances are devices that measure the weight of an object and convert it to a measurement of mass using the acceleration due to gravity. There are two main types of balances:
Triple-Beam Balance
A triple-beam balance is a type of balance that has three movable weights that can be added or removed to balance the weight of an object. The mass of the object is read from a scale that is divided into units, such as grams or ounces.
To use a triple-beam balance, follow these steps:
- Ensure that the balance is level and on a stable surface.
- Adjust the three sliding weights to the desired position, ensuring that the balance is in equilibrium.
- Read the mass of the object from the scale on the balance.
The advantage of a triple-beam balance is that it is relatively simple to use and can measure a wide range of masses. However, it is generally less precise than an electronic balance.
Electronic Balance
An electronic balance is a type of balance that uses electronic sensors to measure the weight of an object and displays the mass on a digital screen. Electronic balances are generally more precise than triple-beam balances and can measure very small masses.
To use an electronic balance, follow these steps:
- Ensure that the balance is level and on a stable surface.
- Turn on the balance and allow it to calibrate.
- Place the object on the balance’s weighing platform.
- Read the mass of the object from the digital display.
The advantage of an electronic balance is its high precision and the ability to measure very small masses. However, electronic balances are generally more expensive than triple-beam balances.
Calculating Mass Using Density and Volume
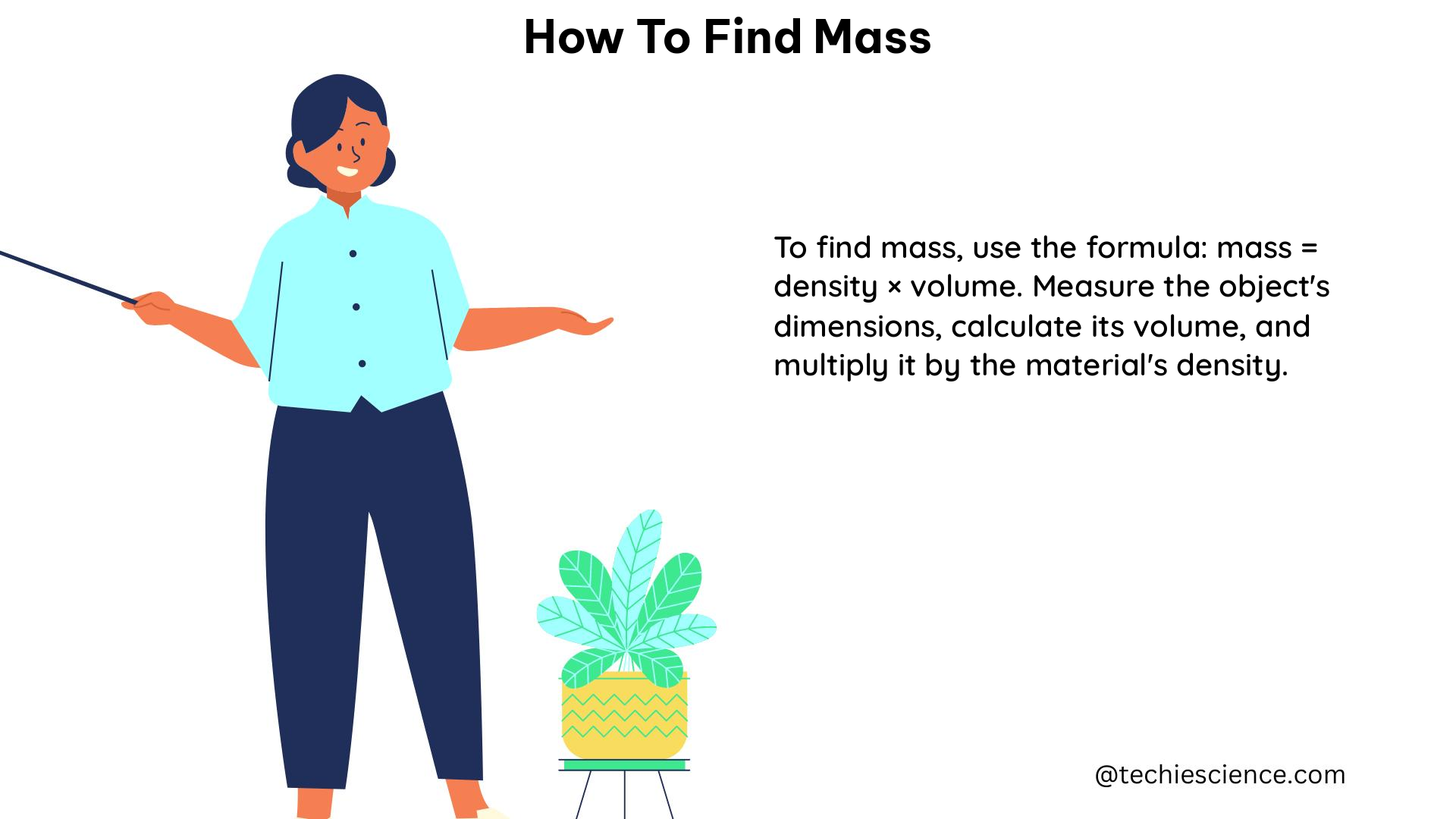
In addition to using a balance, you can also calculate the mass of an object by using the formula for the density of a substance and measuring the volume of the object. The formula for density is:
Density = Mass / Volume
Rearranging this formula, we can solve for the mass:
Mass = Density × Volume
For example, if you have a cube made of aluminum with a side length of 2 cm, you can find the mass of the cube by first calculating the volume of the cube (volume = side length^3 = 2^3 = 8 cm^3) and then multiplying the volume by the density of aluminum (density = 2.7 g/cm^3).
Mass = Density × Volume
Mass = 2.7 g/cm^3 × 8 cm^3
Mass = 21.6 g
Therefore, the mass of the aluminum cube is 21.6 grams.
This method can be particularly useful when dealing with irregularly shaped objects or when the object is too large or heavy to be measured using a balance.
Advanced Mass Measurement Techniques
In addition to the traditional methods of measuring mass using balances and density calculations, there are also more advanced techniques that can be used to measure the mass of objects, particularly in the field of analytical chemistry.
Mass Photometry
Mass photometry is a technique that uses light scattering to measure the mass of individual biomolecules. This method is particularly useful for studying the properties of proteins, nucleic acids, and other biological molecules.
In mass photometry, a laser beam is used to illuminate a sample of the biomolecule, and the scattered light is detected and analyzed to determine the mass of the individual molecules. This technique is highly sensitive and can measure the mass of molecules with a precision of up to 1%.
Accurate Mass Measurement
Accurate mass measurement is an important concept in analytical chemistry, where the precise determination of the mass of a substance is crucial for identifying and quantifying chemical compounds.
There are several factors that can affect the accuracy of mass measurements, including the calibration of the balance, the purity of the sample, and the environmental conditions (such as temperature and humidity) in which the measurement is performed.
To ensure accurate mass measurements, it is important to follow a standardized protocol and to use appropriate statistical methods to analyze the data. This may include techniques such as replicate measurements, uncertainty analysis, and the use of reference materials.
Numerical Examples and Problems
- Example 1: A cube-shaped object made of aluminum has a side length of 5 cm. Calculate the mass of the object.
Given:
– Side length of the cube = 5 cm
– Density of aluminum = 2.7 g/cm^3
Step 1: Calculate the volume of the cube.
Volume = side length^3
Volume = 5 cm × 5 cm × 5 cm = 125 cm^3
Step 2: Calculate the mass of the cube using the formula: Mass = Density × Volume.
Mass = 2.7 g/cm^3 × 125 cm^3 = 337.5 g
Therefore, the mass of the aluminum cube with a side length of 5 cm is 337.5 grams.
- Example 2: A cylindrical object has a radius of 2 cm and a height of 10 cm. The object is made of a material with a density of 3.8 g/cm^3. Calculate the mass of the object.
Given:
– Radius of the cylinder = 2 cm
– Height of the cylinder = 10 cm
– Density of the material = 3.8 g/cm^3
Step 1: Calculate the volume of the cylinder.
Volume = π × r^2 × h
Volume = π × (2 cm)^2 × 10 cm = 125.66 cm^3
Step 2: Calculate the mass of the cylinder using the formula: Mass = Density × Volume.
Mass = 3.8 g/cm^3 × 125.66 cm^3 = 477.51 g
Therefore, the mass of the cylindrical object is 477.51 grams.
-
Problem 1: A rectangular block has dimensions of 4 cm × 6 cm × 8 cm. The block is made of a material with a density of 2.5 g/cm^3. Calculate the mass of the block.
-
Problem 2: A spherical object has a diameter of 10 cm. The object is made of a material with a density of 4.2 g/cm^3. Calculate the mass of the object.
-
Problem 3: A sample of a substance has a mass of 25 grams and occupies a volume of 10 cm^3. Calculate the density of the substance.
These examples and problems demonstrate the application of the concepts and formulas discussed in this guide to calculate the mass of objects using their dimensions and the density of the material.
Conclusion
In this comprehensive guide, we have explored the various methods and techniques used to determine the mass of an object, including the use of balances, density calculations, and advanced mass measurement techniques. By understanding the fundamental concepts of mass and weight, as well as the practical applications of these methods, you will be equipped with the knowledge and tools necessary to accurately measure the mass of any object.
Whether you are a beginner or an experienced physics student, this guide has provided you with a detailed and technical understanding of how to find the mass of an object. By following the step-by-step instructions, solving numerical examples, and applying the provided formulas, you will be able to confidently and accurately measure the mass of any object you encounter.
Remember, the accurate measurement of mass is a crucial aspect of physics and is essential for understanding various physical phenomena. By mastering the techniques outlined in this guide, you will be well on your way to becoming a proficient and knowledgeable physics student.
References
- Webinar: Measuring molecules with light by Prof. Philipp Kukura, University of Oxford
- Measurements and Calculations – SharpSchool
- Accurate Mass Measurement: Terminology and Treatment of Data

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.