Internal energy is a fundamental concept in thermodynamics that describes the total energy of a system, including the kinetic energy of its particles and the potential energy of their interactions. Understanding how to calculate and measure internal energy is crucial for physics students, as it is a key component in understanding energy transfer, work, heat, and entropy. In this comprehensive guide, we will delve into the various methods and techniques for finding internal energy, providing you with a detailed and practical understanding of this important topic.
Calculating Internal Energy of a Gas
The internal energy of a gas can be calculated using the equation:
U = 1.5 N k_b T = 1.5 n R T
Where:
– U is the internal energy of the gas
– N is the number of particles in the gas
– k_b is the Boltzmann constant (1.38 × 10^-23 J/K)
– T is the absolute temperature of the gas in Kelvin
– n is the number of moles of the gas
– R is the universal gas constant (8.314 J/mol·K)
For example, if you have a gas with 56,000 Joules of internal energy, 1 mole of particles, and a temperature of 300 Kelvin, you can calculate the pressure of the gas using the ideal gas law:
PV = nRT
Where:
– P is the pressure of the gas
– V is the volume of the gas
Once you have the pressure, you can then use the equation U = 1.5 n RT to find the temperature or the volume of the gas, if you know the other variables.
Measuring Internal Energy Using Calorimetry
Calorimetry is the method of measuring the heat absorbed or released by a system during a physical or chemical change. This technique can be used to measure the internal energy of a substance.
To measure the specific internal energy of a substance, you can follow these steps:
- Place a known mass of the substance in a calorimeter.
- Heat the substance by a known amount of energy (e.g., by passing an electric current through a heating element).
- Measure the temperature change of the substance and the surrounding environment.
- Use the formula
q = m c ΔTto calculate the specific internal energy of the substance, where: qis the amount of heat absorbed or releasedmis the mass of the substancecis the specific heat capacity of the substanceΔTis the change in temperature
The specific internal energy is the internal energy per unit mass or mole of the substance, and it can be expressed in Joules per gram or Joules per mole.
Understanding Internal Energy as a State Function
Internal energy is a state function, which means that it depends only on the current state of the system and not on the path or process that led to that state. This concept is important for understanding the behavior of thermodynamic systems.
State functions and state variables are properties that describe the equilibrium state of a system, such as temperature, pressure, volume, and composition. Any change in these variables implies a change in the state of the system, and the internal energy is directly related to these state variables.
For example, if you change the temperature, pressure, or volume of a system, the internal energy of the system will also change. This relationship is described by the fundamental thermodynamic equation:
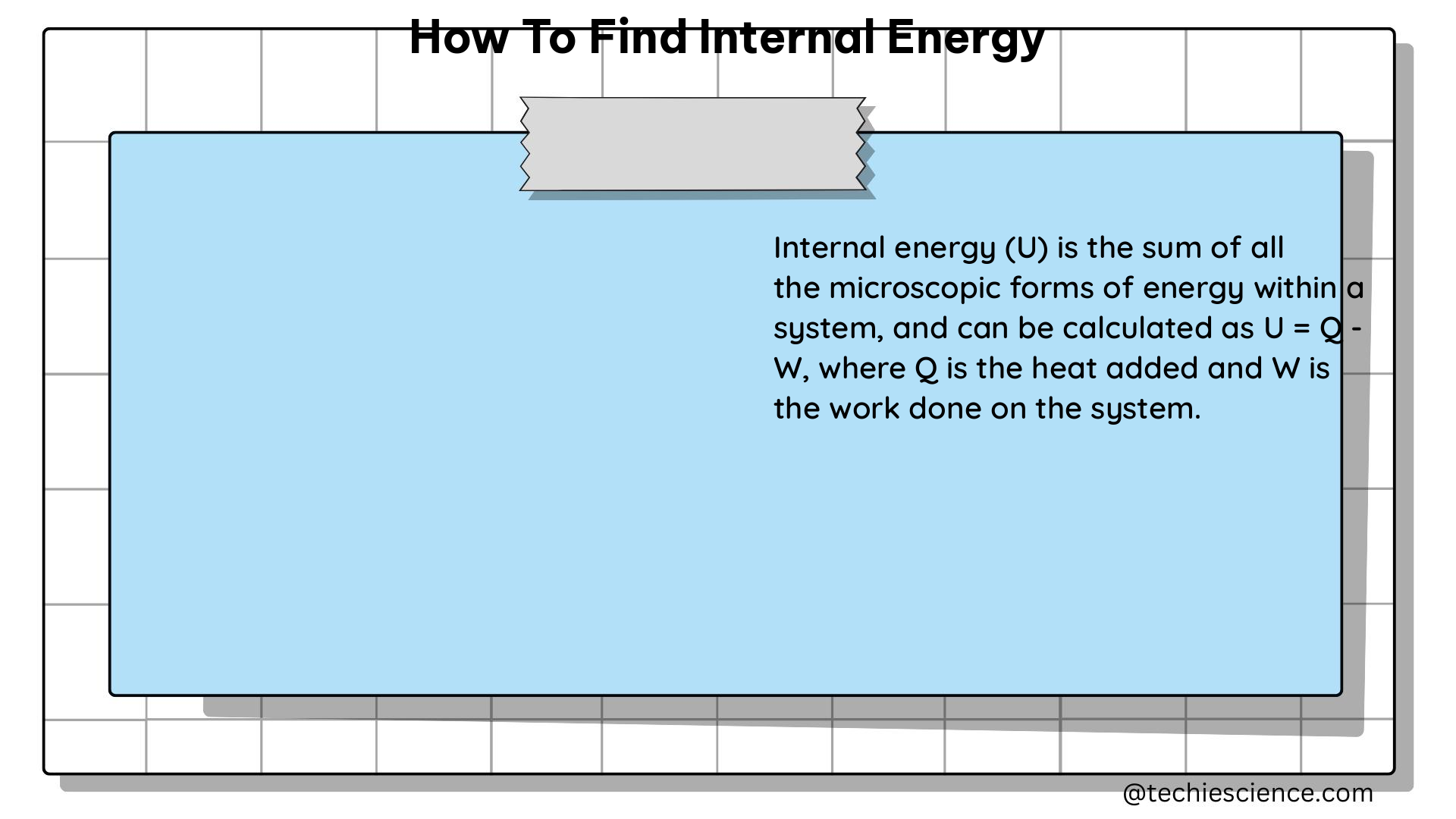
dU = dq + dw
Where:
– dU is the change in internal energy
– dq is the heat added to the system
– dw is the work done on the system
By understanding the internal energy as a state function, you can use the relationships between state variables to calculate and predict the behavior of thermodynamic systems.
Advanced Techniques for Finding Internal Energy
In addition to the basic methods discussed above, there are more advanced techniques for finding internal energy that may be useful for physics students:
-
Quantum Mechanical Approach: The internal energy of a system can be calculated using quantum mechanical principles, which take into account the discrete energy levels of the particles in the system. This approach is particularly useful for understanding the internal energy of solids, liquids, and quantum systems.
-
Statistical Mechanics Approach: The internal energy of a system can also be calculated using statistical mechanics, which involves the statistical distribution of the energy among the particles in the system. This approach is useful for understanding the behavior of large systems with many particles, such as gases and solids.
-
Molecular Dynamics Simulations: Computer simulations of molecular dynamics can be used to calculate the internal energy of a system by tracking the motion and interactions of individual particles. This approach is useful for understanding the behavior of complex systems, such as biological molecules or materials with complex structures.
-
Experimental Techniques: In addition to calorimetry, there are other experimental techniques for measuring internal energy, such as neutron scattering, X-ray diffraction, and nuclear magnetic resonance (NMR) spectroscopy. These techniques can provide detailed information about the structure and dynamics of a system, which can be used to infer its internal energy.
By understanding these advanced techniques, physics students can gain a deeper and more comprehensive understanding of internal energy and its role in the behavior of physical systems.
Conclusion
In this comprehensive guide, we have explored the various methods and techniques for finding internal energy, from the basic equations for calculating the internal energy of a gas to the more advanced approaches using quantum mechanics, statistical mechanics, and experimental techniques. By mastering these concepts, physics students can develop a strong foundation in thermodynamics and apply their knowledge to a wide range of physical systems and phenomena.
Remember, the key to understanding internal energy is to focus on the relationships between state variables and the fundamental thermodynamic principles that govern the behavior of these systems. With practice and a deep understanding of these concepts, you will be well on your way to becoming a proficient physicist.
References
- How to Calculate the Internal Energy of a Thermodynamic System: https://study.com/skill/learn/how-to-calculate-the-internal-energy-of-a-thermodynamic-system-explanation.html
- Specific Internal Energy: https://www.sciencedirect.com/topics/engineering/specific-internal-energy
- Internal Energy as a State of System: https://www.geeksforgeeks.org/internal-energy-as-a-state-of-system/
- Explaining internal energy from a macroscopic perspective: https://physics.stackexchange.com/questions/715019/explaining-internal-energy-from-a-macroscopic-perspective
- Calculating internal energy and work example (video) | Khan Academy: https://www.khanacademy.org/science/chemistry/thermodynamics-chemistry/internal-energy-sal/v/calculating-internal-energy-and-work-example

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.