Summary
Heat energy is a fundamental concept in physics, and understanding how to measure and calculate it is crucial for many applications, from engineering to scientific research. This comprehensive guide will provide you with a detailed understanding of the principles and techniques used to find heat energy, including the formula, specific heat capacity, and the impact of the container’s heat capacity. Whether you’re a physics student or a researcher, this guide will equip you with the knowledge and tools you need to accurately determine heat energy in various scenarios.
Understanding the Heat Energy Formula
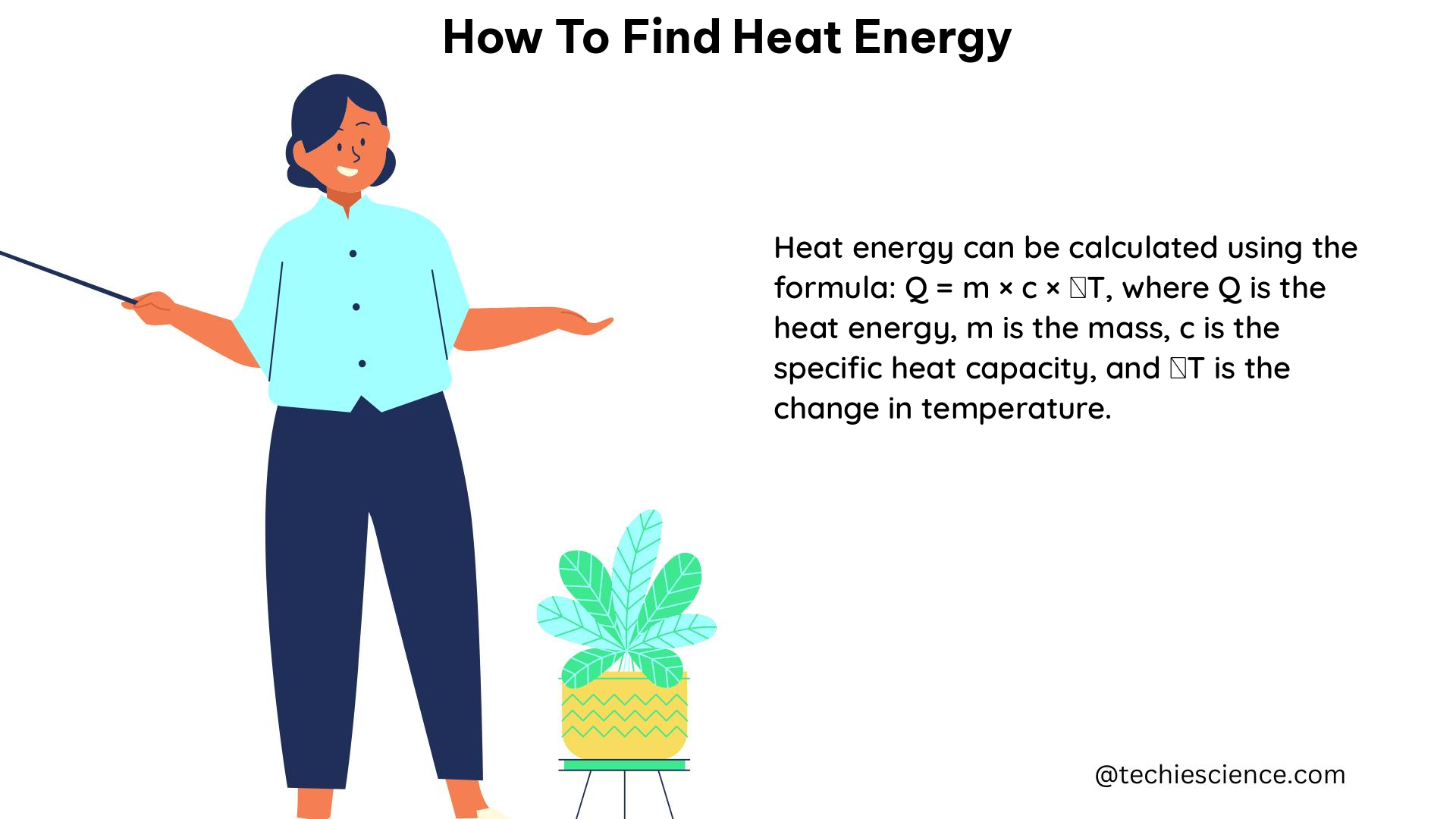
The formula used to find heat energy is:
Q = mcΔT
Where:
– Q is the heat energy (in Joules)
– m is the mass of the object (in grams)
– c is the specific heat capacity of the substance (in J/g°C)
– ΔT is the change in temperature (in degrees Celsius)
The specific heat capacity is the amount of heat energy required to raise the temperature of one gram of a substance by one degree Celsius. This value varies depending on the material, and it’s essential to use the correct value for the substance you’re working with.
Calculating Heat Energy: Examples and Numerical Problems
Example 1: Heating Water
Suppose you want to find the heat energy required to raise the temperature of 50 grams of water by 10 degrees Celsius.
Given:
– Mass (m) = 50 grams
– Specific heat capacity of water (c) = 4.18 J/g°C
– Temperature change (ΔT) = 10°C
Substituting the values into the formula:
Q = mcΔT
Q = (50 g) × (4.18 J/g°C) × (10°C)
Q = 2090 J
Therefore, it would take 2090 Joules of heat energy to raise the temperature of 50 grams of water by 10 degrees Celsius.
Example 2: Heating Aluminum
Now, let’s consider the heat energy required to raise the temperature of 50 grams of aluminum by 10 degrees Celsius.
Given:
– Mass (m) = 50 grams
– Specific heat capacity of aluminum (c) = 0.90 J/g°C
– Temperature change (ΔT) = 10°C
Substituting the values into the formula:
Q = mcΔT
Q = (50 g) × (0.90 J/g°C) × (10°C)
Q = 450 J
In this case, it would take 450 Joules of heat energy to raise the temperature of 50 grams of aluminum by 10 degrees Celsius.
Numerical Problem 1
A calorimeter with a heat capacity of 20 J/°C is used to measure the heat energy absorbed by 100 grams of copper when its temperature is raised from 20°C to 50°C. Calculate the heat energy absorbed by the copper.
Given:
– Mass of copper (m) = 100 grams
– Specific heat capacity of copper (c) = 0.385 J/g°C
– Initial temperature (T1) = 20°C
– Final temperature (T2) = 50°C
– Heat capacity of the calorimeter (Cc) = 20 J/°C
Calculating the temperature change (ΔT):
ΔT = T2 - T1
ΔT = 50°C - 20°C
ΔT = 30°C
Calculating the heat energy absorbed by the copper:
Q = mcΔT + CcΔT
Q = (100 g) × (0.385 J/g°C) × (30°C) + (20 J/°C) × (30°C)
Q = 1155 J + 600 J
Q = 1755 J
Therefore, the heat energy absorbed by the copper is 1755 Joules.
Numerical Problem 2
A 50-gram sample of an unknown substance is heated from 20°C to 50°C, and it is found that 2500 Joules of heat energy are required. Determine the specific heat capacity of the unknown substance.
Given:
– Mass of the substance (m) = 50 grams
– Initial temperature (T1) = 20°C
– Final temperature (T2) = 50°C
– Heat energy absorbed (Q) = 2500 Joules
Calculating the temperature change (ΔT):
ΔT = T2 - T1
ΔT = 50°C - 20°C
ΔT = 30°C
Rearranging the heat energy formula to solve for the specific heat capacity (c):
Q = mcΔT
c = Q / (mΔT)
c = 2500 J / (50 g × 30°C)
c = 1.67 J/g°C
Therefore, the specific heat capacity of the unknown substance is 1.67 J/g°C.
The Impact of the Container’s Heat Capacity
When measuring heat energy, it’s essential to consider the heat capacity of the container or calorimeter used to measure the heat transfer. The heat capacity is the amount of heat energy required to raise the temperature of the container by one degree Celsius.
The formula to account for the heat capacity of the container is:
Q = mcΔT + CcΔTc
Where:
– Cc is the heat capacity of the container (in J/°C)
– ΔTc is the change in temperature of the container (in degrees Celsius)
By including the heat capacity of the container in the calculation, you can obtain a more accurate measurement of the heat energy absorbed or released by the substance of interest.
Additional Considerations and Data Points
Specific Heat Capacity of Common Substances
Here is a table of the specific heat capacities of some common substances:
| Substance | Specific Heat Capacity (J/g°C) |
|---|---|
| Water | 4.18 |
| Aluminum | 0.90 |
| Copper | 0.385 |
| Iron | 0.449 |
| Glass | 0.840 |
| Ethanol | 2.44 |
| Concrete | 0.880 |
Conversion of Heat Energy Units
Heat energy can be measured in various units, and it’s important to be aware of the conversions between them:
- 1 Joule (J) = 1 N·m = 0.239 calories (cal)
- 1 calorie (cal) = 4.184 Joules (J)
- 1 kilowatt-hour (kWh) = 3,600,000 Joules (J)
Factors Affecting Specific Heat Capacity
The specific heat capacity of a substance can be influenced by several factors, including:
- Temperature: The specific heat capacity can vary slightly with temperature, especially at extreme temperatures.
- Pressure: Changes in pressure can also affect the specific heat capacity, particularly for gases.
- Phase changes: During phase changes, such as melting or boiling, the specific heat capacity can change significantly.
- Molecular structure: The molecular structure and bonding of a substance can influence its specific heat capacity.
Understanding these factors can help you make more accurate calculations and interpretations when working with heat energy.
Conclusion
Measuring and calculating heat energy is a fundamental skill in physics, with applications in various fields. By understanding the heat energy formula, the specific heat capacity of different substances, and the impact of the container’s heat capacity, you can accurately determine the heat energy involved in various processes and scenarios. This comprehensive guide has provided you with the necessary knowledge and tools to become proficient in finding heat energy, equipping you with the skills to tackle complex problems and make informed decisions in your studies or research.
References:
- Specific Heat Capacity of Common Substances
- Conversion of Heat Energy Units
- Factors Affecting Specific Heat Capacity

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.