In the realm of physics, understanding the relationship between energy and frequency is a fundamental concept that holds the key to unraveling the mysteries of the universe. Whether you’re a student, a researcher, or simply someone curious about the intricacies of the physical world, this comprehensive guide will equip you with the knowledge and tools to navigate the intricate dance between energy and frequency.
Unveiling the Formula: E = hf
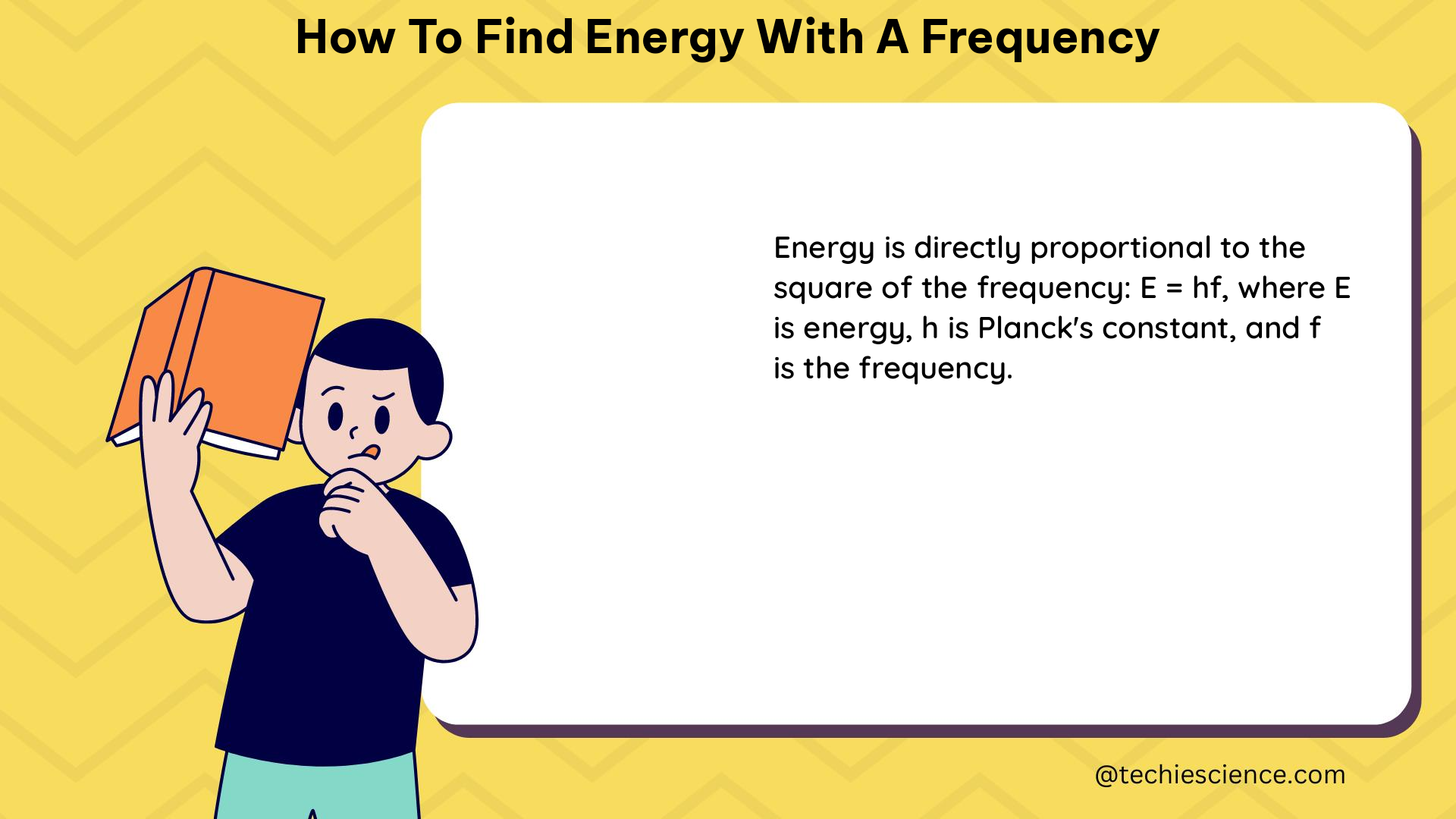
The cornerstone of understanding the energy associated with a wave of a given frequency lies in the formula E = hf, where E represents the energy, h is Planck’s constant (6.63 × 10^-34 Js), and f is the frequency. This equation, derived from the groundbreaking work of Max Planck, allows us to calculate the energy of a photon, the fundamental particle of light, based on its frequency.
Applying the Formula: Photon Energy Calculations
Let’s consider a practical example to illustrate the application of the E = hf formula. Imagine we have a red laser with a frequency of 4.5 × 10^14 Hz. To determine the energy of a single photon in this laser, we can simply plug the values into the equation:
E = (6.63 × 10^-34 Js) × (4.5 × 10^14 Hz)
E = 2.98 × 10^-19 J
This calculation reveals that the energy of a single photon in the red laser is a mere 2.98 × 10^-19 Joules, a testament to the minuscule scale of quantum phenomena.
Exploring Sound Wave Energy
While the E = hf formula is primarily used for light waves, the energy of sound waves can be calculated using a different approach. In the case of sound waves, the energy is proportional to the square of the amplitude and the square of the frequency. This means that a sound wave with a higher amplitude or frequency will possess more energy than a wave with a lower amplitude or frequency.
Calculating Sound Wave Energy
Let’s consider two sound waves, one with an amplitude of 1 Pa and a frequency of 100 Hz, and another with an amplitude of 2 Pa and a frequency of 200 Hz. We can use the formula E = p^2 × f, where p is the amplitude and f is the frequency, to calculate the energy of each wave.
For the first wave:
E = (1 Pa)^2 × (100 Hz)
E = 100 Pa^2 Hz
For the second wave:
E = (2 Pa)^2 × (200 Hz)
E = 800 Pa^2 Hz
The calculations reveal that the second wave has four times the energy of the first wave, a direct consequence of its higher amplitude and frequency.
Quantum Energy Levels and Transitions
In the realm of quantum mechanics, the energy of a particle or system is quantized, meaning it can only take on discrete, specific values. This concept is particularly important in understanding the energy levels and transitions of atoms and molecules.
Bohr’s Model and Atomic Energy Levels
Niels Bohr’s groundbreaking model of the atom describes the electron as orbiting the nucleus in specific, allowed energy levels. The energy of an electron in a particular level is determined by the formula E = -13.6 eV / n^2, where n is the principal quantum number representing the energy level.
When an electron transitions from a higher energy level to a lower energy level, it releases a photon with an energy equal to the difference between the two levels. This energy is directly proportional to the frequency of the emitted photon, as described by the E = hf formula.
Quantum Tunneling and Energy Barriers
In the quantum realm, particles can exhibit the phenomenon of tunneling, where they can overcome energy barriers that would be classically forbidden. This process is governed by the wave-like nature of particles and the uncertainty principle, which allows for the possibility of particles “tunneling” through barriers with energies lower than the barrier height.
The probability of tunneling is directly related to the energy difference between the particle and the barrier, as well as the width of the barrier. Understanding this concept is crucial in fields such as semiconductor physics, where quantum tunneling plays a vital role in the operation of devices like tunnel diodes and scanning tunneling microscopes.
Practical Applications and Implications
The relationship between energy and frequency has far-reaching implications in various fields of science and technology. From the design of communication systems to the development of advanced materials, the ability to harness and manipulate energy and frequency is a cornerstone of modern scientific and engineering endeavors.
Telecommunications and Wireless Communication
In the realm of telecommunications, the frequency spectrum is a valuable resource that is carefully managed and utilized. The energy and frequency of electromagnetic waves are the foundation of wireless communication technologies, such as radio, television, and cellular networks. By understanding the relationship between energy and frequency, engineers can optimize the efficiency and performance of these systems.
Spectroscopy and Material Analysis
The study of the interaction between matter and electromagnetic radiation, known as spectroscopy, relies heavily on the principles of energy and frequency. By analyzing the absorption, emission, or scattering of light by materials, scientists can gain valuable insights into the atomic and molecular structure of substances, enabling applications in fields like chemistry, materials science, and astrophysics.
Energy Generation and Storage
The conversion of energy between different forms, such as electrical, mechanical, and thermal, is a fundamental aspect of energy generation and storage technologies. Understanding the relationship between energy and frequency is crucial in the design and optimization of systems like power generators, batteries, and energy storage devices.
Quantum Computing and Information Processing
The emerging field of quantum computing and information processing is heavily dependent on the principles of quantum mechanics, including the quantization of energy levels and the wave-like behavior of particles. By harnessing the unique properties of quantum systems, researchers are working to develop revolutionary computing and communication technologies that could far surpass the capabilities of classical systems.
Conclusion
In the captivating world of physics, the relationship between energy and frequency is a cornerstone of our understanding of the physical universe. From the microscopic realm of quantum mechanics to the vast expanse of the cosmos, the ability to harness and manipulate energy and frequency has been a driving force behind scientific and technological advancements.
By delving into the intricacies of the E = hf formula, exploring the energy dynamics of sound waves, and examining the quantum-level phenomena, this comprehensive guide has provided you with the tools and knowledge to navigate the intricate dance between energy and frequency. As you continue your journey of discovery, may this guide serve as a valuable resource, empowering you to unlock the secrets of the physical world and contribute to the ongoing quest for scientific understanding.
References:
– Calculating the Energy of a Light Wave Given Frequency – Study.com
– Calculating power and energy based on frequency and amplitude … – Physics Forums
– How to Calculate the Quantum Energy of LIght | Physics – Study.com
– How to Convert Frequency to Energy – YouTube
– 16.4 Energy and Power of a Wave – University Physics Volume 1

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.