Energy can be transformed from one form to another, and one of the most common transformations is the conversion of energy into heat. Understanding how to quantify this energy-to-heat conversion is crucial for various applications, such as in engineering, thermodynamics, and energy efficiency analysis. In this comprehensive guide, we will explore the fundamental principles and practical methods for finding the energy turned into heat.
Understanding Heat Transfer and the Q = mcΔT Formula
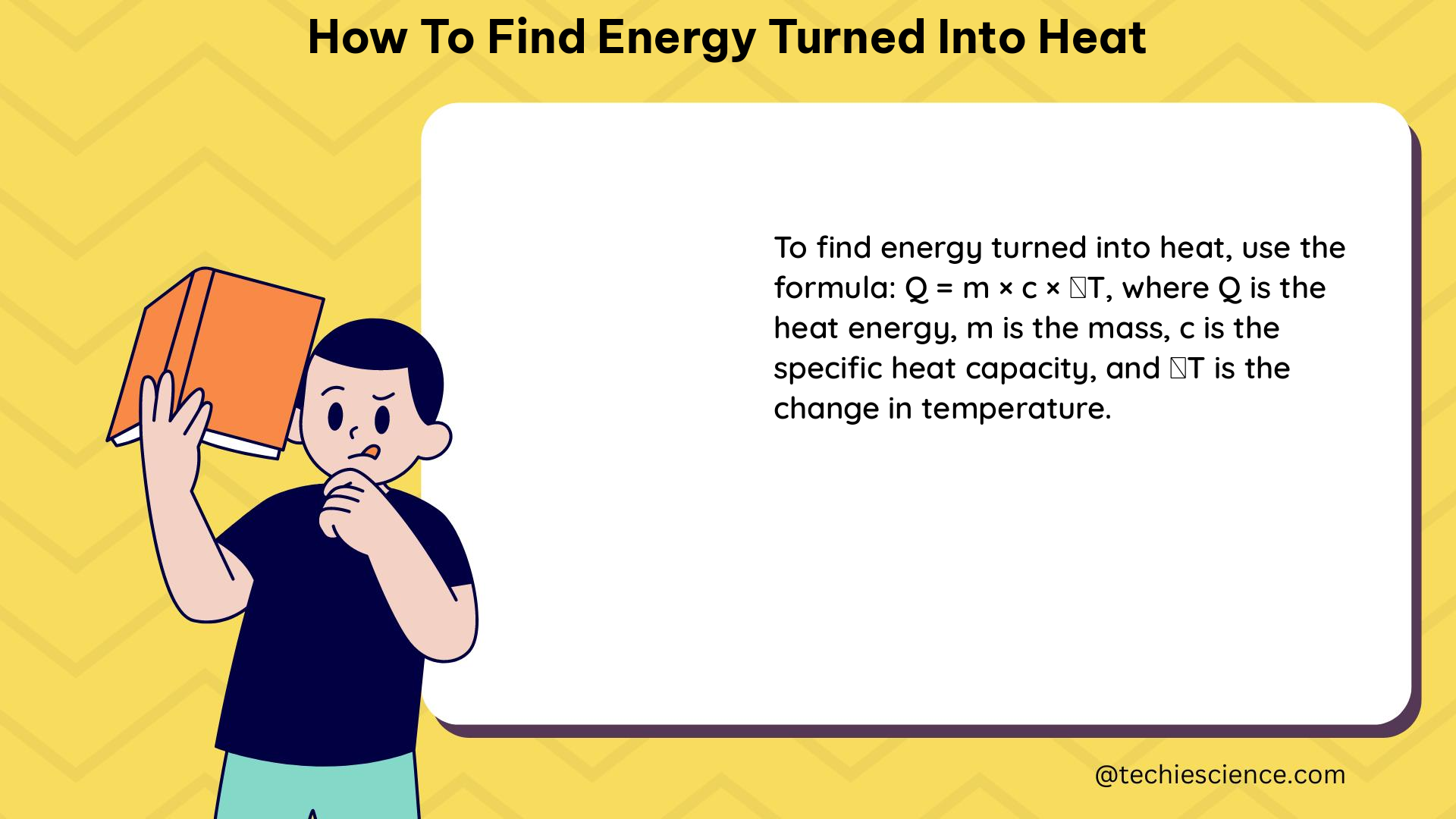
The primary equation used to calculate the energy turned into heat is the heat transfer formula, Q = mcΔT, where:
- Q is the heat transferred (in Joules)
- m is the mass of the object (in kilograms)
- c is the specific heat capacity of the material (in Joules per kilogram-degree Celsius)
- ΔT is the change in temperature (in degrees Celsius)
This formula allows us to determine the amount of heat transferred when an object’s temperature changes. Let’s consider an example:
Suppose we have a 500-gram piece of aluminum at a temperature of 100°C, and we want to find the heat transferred when it cools down to 25°C. The specific heat capacity of aluminum is 0.90 J/g°C.
First, we calculate the change in temperature:
ΔT = Tfinal – Tinitial
ΔT = 25°C – 100°C = -75°C
Next, we plug the values into the Q = mcΔT formula:
Q = (500 g) × (0.90 J/g°C) × (-75°C)
Q = -33,750 J
The negative sign indicates that heat is leaving the aluminum and being transferred to the surroundings.
Applying the Work-Energy Theorem to Find Energy Turned into Heat
In more advanced scenarios, where mechanical work is involved, we can use the work-energy theorem to determine the energy turned into heat. The work-energy theorem states that the net work done on an object is equal to its change in kinetic energy.
Let’s consider an example:
Suppose we have a metal cylinder with a mass of 2 kg, and we turn it against a frictional force of 50 N. The cylinder is turned 100 times, and the circumference of the cylinder is 0.5 meters.
First, we can calculate the work done by the frictional force:
W = Fd
W = (50 N) × (0.5 m) × (100)
W = 2,500 J
Next, we can use the work-energy theorem to find the energy turned into heat:
W = ΔE
2,500 J = ΔE
Assuming that all the mechanical work is used to increase the internal energy and raise the temperature of the cylinder, we can write the work in terms of temperature:
W = A ΔT
2,500 J = A × (ΔT)
where A is a constant that represents the amount of work (in Joules) necessary to raise the temperature by 1°C.
To find the value of A, we can use the heat transfer formula:
Q = mcΔT
Q = (2 kg) × (0.90 J/kg°C) × (ΔT)
Q = 1.80 ΔT J
Since W = Q, we can set them equal to each other and solve for A:
2,500 J = 1.80 ΔT J
A = 2,500 J / ΔT
The value of A depends on the specific heat capacity of the cylinder material, which can be looked up in a handbook.
Advanced Techniques for Calculating Energy Turned into Heat
In addition to the basic heat transfer formula and the work-energy theorem, there are more advanced techniques and considerations for calculating the energy turned into heat:
-
Calorimetry: Calorimetry is the experimental study of the heat exchange in a chemical or physical process. By using calorimetric techniques, such as bomb calorimetry or differential scanning calorimetry, you can directly measure the heat released or absorbed during a process.
-
Thermodynamic Principles: Applying the principles of thermodynamics, such as the first law of thermodynamics and the concept of internal energy, can provide a deeper understanding of the energy transformations and the relationship between work, heat, and internal energy.
-
Phase Changes and Latent Heat: When a substance undergoes a phase change, such as melting or boiling, the energy required or released during the phase change is known as latent heat. Accounting for latent heat is essential when calculating the energy turned into heat in phase change processes.
-
Thermal Efficiency and Energy Conversion Processes: In the context of energy conversion systems, such as heat engines or power plants, the concept of thermal efficiency can be used to quantify the fraction of the input energy that is converted into useful work, with the remaining energy being turned into heat.
-
Numerical Simulations and Computational Fluid Dynamics (CFD): Advanced computational techniques, such as CFD, can be employed to model and simulate complex heat transfer and energy conversion processes, allowing for more accurate predictions of the energy turned into heat.
-
Experimental Measurements and Instrumentation: Precise experimental measurements using various instruments, such as thermocouples, calorimeters, and heat flux sensors, can provide valuable data for calculating the energy turned into heat in real-world scenarios.
By understanding and applying these advanced techniques, you can gain a more comprehensive understanding of the energy-to-heat conversion process and accurately quantify the energy turned into heat in a wide range of applications.
Conclusion
In this comprehensive guide, we have explored the fundamental principles and practical methods for finding the energy turned into heat. From the basic heat transfer formula, Q = mcΔT, to the more advanced work-energy theorem and thermodynamic considerations, we have provided a detailed overview of the key concepts and techniques involved in this important area of physics and engineering.
By mastering these methods, you will be equipped to tackle a wide range of problems and scenarios related to energy conversion and heat transfer, enabling you to make informed decisions and optimize the efficiency of various systems and processes.
Remember, the accurate quantification of energy turned into heat is crucial for applications ranging from thermal management and energy efficiency to the design of heat engines and power plants. By applying the principles and techniques outlined in this guide, you can become a true expert in this field and contribute to the advancement of science and technology.
References
- Depth Home, Brooklyn College. “Mechanical Work, Energy Transfer to Heat.” http://depthome.brooklyn.cuny.edu/physics/lab/phy1/Mechanical-work-energy-transfer-to-heat-final.pdf
- The Physics Classroom. “Measuring the Quantity of Heat.” https://www.physicsclassroom.com/class/thermalP/Lesson-2/Measuring-the-Quantity-of-Heat
- Lumen Learning. “Temperature Change and Heat Capacity.” https://courses.lumenlearning.com/suny-physics/chapter/14-2-temperature-change-and-heat-capacity/

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.