Nuclear reactions are fundamental processes that power various applications, from nuclear energy generation to medical diagnostics. Understanding the energy released in these reactions is crucial for understanding the underlying physics and optimizing their practical applications. In this comprehensive guide, we will delve into the step-by-step process of calculating the energy produced in nuclear reactions.
1. Calculate the Mass Defect
The first step in determining the energy produced in a nuclear reaction is to calculate the mass defect. The mass defect is the difference between the sum of the masses of the reactants and the sum of the masses of the products.
To calculate the mass defect, follow these steps:
- Identify the reactants and products involved in the nuclear reaction.
- Determine the masses of each reactant and product in unified atomic mass units (amu).
- Add up the masses of all the reactants.
- Add up the masses of all the products.
- Subtract the sum of the product masses from the sum of the reactant masses to obtain the mass defect.
The mass defect can be expressed mathematically as:
Mass Defect = Σ(Masses of Reactants) - Σ(Masses of Products)
2. Convert Mass Defect to Energy
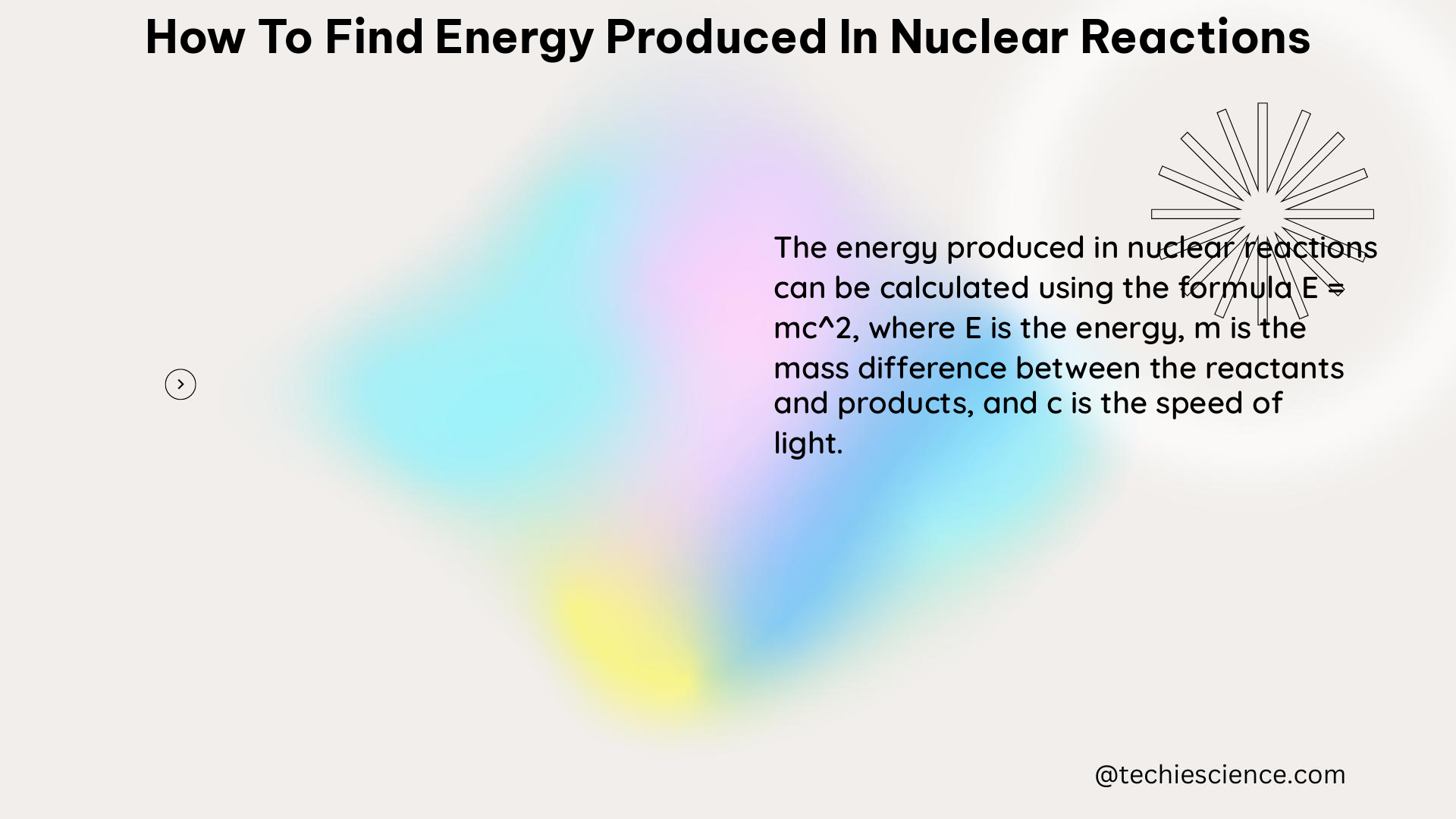
Once the mass defect has been calculated, the next step is to convert it to the corresponding energy released in the nuclear reaction. This is done using Einstein’s mass-energy equivalence equation:
E = mc^2
where:
– E is the energy released (in joules)
– m is the mass defect (in kilograms)
– c is the speed of light (3 × 10^8 m/s)
To convert the mass defect from amu to kilograms, you can use the conversion factor:
1 amu = 1.660539 × 10^-27 kg
Once you have the mass defect in kilograms, simply plug it into the mass-energy equivalence equation to calculate the energy released.
3. Scale Up for One Mole
If you want to find the energy released per mole of reactants, you can multiply the energy released for one nucleus by the Avogadro constant:
Energy Released per Mole = Energy Released per Nucleus × Avogadro Constant
where the Avogadro constant is 6.022 × 10^23 particles/mol.
Example Calculations
Let’s apply the steps outlined above to two common nuclear reactions: fusion and fission.
Fusion Reaction: 2H + 3H → 4He + 1n
- Calculate the Mass Defect:
- Reactant masses: 2H = 2.0141 amu, 3H = 3.0160 amu
- Product masses: 4He = 4.0026 amu, 1n = 1.0087 amu
-
Mass defect = (2.0141 + 3.0160) – (4.0026 + 1.0087) = 0.0198 amu
-
Convert Mass Defect to Energy:
- Convert mass defect to kilograms: 0.0198 amu × (1 kg / 6.022 × 10^23 amu) = 3.23 × 10^-29 kg
-
Energy released: E = mc^2 = 3.23 × 10^-29 kg × (3 × 10^8 m/s)^2 = 2.91 × 10^-12 J
-
Scale Up for One Mole:
- Energy released per mole: 2.91 × 10^-12 J × 6.022 × 10^23 = 1.75 × 10^9 J/mol
Fission Reaction: 235U → 141Ba + 92Kr + 3 1n
- Calculate the Mass Defect:
- Reactant mass: 235U = 235.0439 amu
- Product masses: 141Ba = 140.9144 amu, 92Kr = 91.9262 amu, 1n = 1.0087 amu
-
Mass defect = 235.0439 – (140.9144 + 91.9262 + 3 × 1.0087) = 0.1858 amu
-
Convert Mass Defect to Energy:
- Convert mass defect to kilograms: 0.1858 amu × (1 kg / 6.022 × 10^23 amu) = 3.08 × 10^-28 kg
-
Energy released: E = mc^2 = 3.08 × 10^-28 kg × (3 × 10^8 m/s)^2 = 2.78 × 10^11 J
-
Scale Up for One Mole:
- Energy released per mole: 2.78 × 10^11 J × 6.022 × 10^23 = 1.67 × 10^10 J/mol
Key Formulas and Values
- Einstein’s Mass-Energy Equivalence:
E = mc^2 c = 3 × 10^8 m/s(speed of light)1 amu = 1.660539 × 10^-27 kg(conversion factor)-
1 MeV = 1.602176 × 10^-13 J(conversion factor) -
Avogadro Constant:
6.022 × 10^23 particles/mol
References
- Purdue University. (n.d.). Determining the Energy Change of a Nuclear Reaction. Retrieved from https://www.chem.purdue.edu/gchelp/howtosolveit/Nuclear/Energy_of_Nuclear_Change.htm
- AstroNuclPhysics. (n.d.). Nuclear reactions and nuclear energy. Retrieved from https://astronuclphysics.info/JadRadFyzika3.htm
- LibreTexts. (2020). 21.6: Energy Changes in Nuclear Reactions. Retrieved from https://chem.libretexts.org/Courses/University_of_Missouri/MU:__1330H_%28Keller%29/21:_Nuclear_Chemistry/21.6:_Energy_Changes_in_Nuclear_Reactions
- YouTube. (2016). C.7 Calculating energy released in nuclear reactions (HL). Retrieved from https://www.youtube.com/watch?v=XzcgTbl8ClU
- ScienceFlip. (n.d.). Quantitative Analysis of Nuclear Reactions. Retrieved from https://www.scienceflip.com.au/subjects/physics/fromtheuniversetotheatom/learn13/

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.