Summary
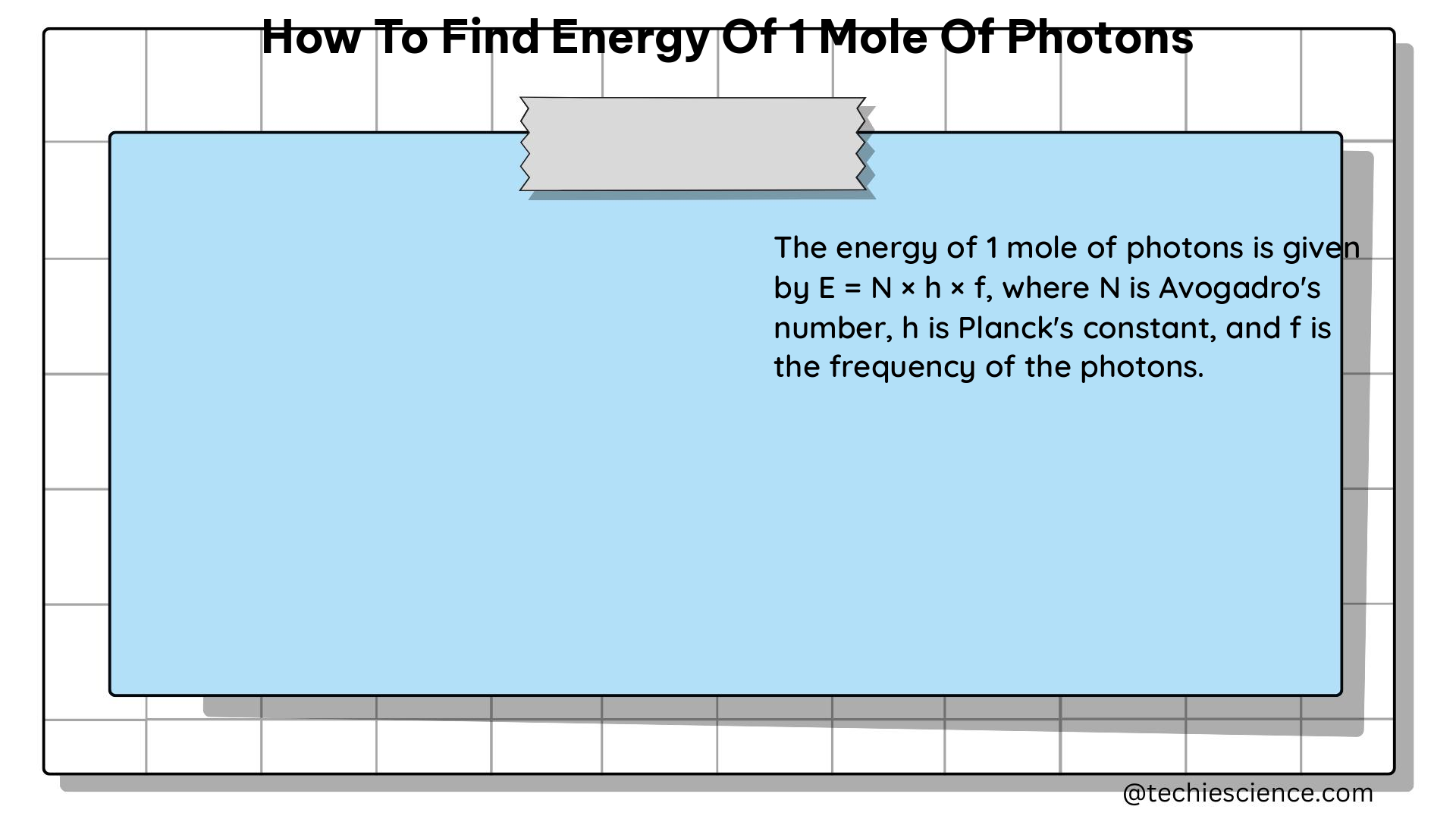
Determining the energy of one mole of photons involves several key steps, including identifying the wavelength or frequency of the light, calculating the energy of a single photon, and then multiplying that value by Avogadro’s number to find the total energy. This guide provides a detailed, technical walkthrough of the entire process, complete with relevant physics formulas, examples, and data points to help physics students master this concept.
Understanding Photon Energy
The energy of a photon is directly related to its wavelength or frequency, as described by the equation:
E = hc/λ
Where:
– E is the energy of the photon (in Joules)
– h is Planck’s constant (6.63 × 10^-34 J·s)
– c is the speed of light (3.0 × 10^8 m/s)
– λ is the wavelength of the photon (in meters)
This relationship means that photons with shorter wavelengths (or higher frequencies) have higher energy levels. For example, a photon with a wavelength of 500 nm will have less energy than a photon with a wavelength of 400 nm.
Calculating the Energy of 1 Mole of Photons
To find the energy of one mole of photons, we’ll need to follow these steps:
-
Identify the Wavelength or Frequency: Start by determining the wavelength or frequency of the photons you’re working with. This information is typically provided in the problem statement. If the wavelength is given in nanometers (nm), convert it to meters (m) by multiplying by 10^-9.
-
Calculate the Energy of a Single Photon: Use the formula E = hc/λ to calculate the energy of a single photon. Plug in the appropriate values for Planck’s constant (h), the speed of light (c), and the wavelength (λ).
-
Multiply by Avogadro’s Number: To find the energy of one mole of photons, multiply the energy of a single photon by Avogadro’s number (6.02 × 10^23 mol^-1). This gives you the total energy contained in one mole of photons.
Let’s walk through an example to illustrate the process:
Example: Calculating the Energy of 1 Mole of Photons with a Frequency of 5 × 10^14 Hz
Step 1: Identify the Frequency
The problem statement gives the frequency of the photons as 5 × 10^14 Hz.
Step 2: Calculate the Energy of a Single Photon
Using the formula E = hc/λ, we can calculate the energy of a single photon:
– h = Planck’s constant = 6.626 × 10^-34 J·s
– c = speed of light = 3.0 × 10^8 m/s
– λ = wavelength = c/v = 3.0 × 10^8 m/s / 5 × 10^14 Hz = 6.0 × 10^-7 m
– E = hc/λ = (6.626 × 10^-34 J·s)(3.0 × 10^8 m/s) / (6.0 × 10^-7 m) = 3.313 × 10^-19 J
Step 3: Multiply by Avogadro’s Number
To find the energy of one mole of photons, we multiply the energy of a single photon by Avogadro’s number:
– Energy of 1 mole of photons = (3.313 × 10^-19 J) × (6.02 × 10^23 mol^-1) = 199.51 kJ/mol
Therefore, the energy of one mole of photons with a frequency of 5 × 10^14 Hz is 199.51 kJ/mol.
Additional Examples and Considerations
Example: Calculating the Energy of 1 Mole of Photons with a Wavelength of 500 nm
- Wavelength (λ) = 500 nm = 5.0 × 10^-7 m
- Energy of a single photon, E = hc/λ = (6.63 × 10^-34 J·s)(3.0 × 10^8 m/s) / (5.0 × 10^-7 m) = 3.9 × 10^-19 J
- Energy of 1 mole of photons = (3.9 × 10^-19 J) × (6.02 × 10^23 mol^-1) = 234.78 kJ/mol
Considerations
- The energy of a photon is quantized, meaning it can only take on certain discrete values. This is a fundamental property of quantum mechanics.
- The relationship between photon energy and wavelength/frequency is inversely proportional, so higher-energy photons have shorter wavelengths and higher frequencies.
- Photon energy is an important concept in various fields, such as spectroscopy, quantum optics, and the study of atomic and molecular structure.
Conclusion
Determining the energy of one mole of photons is a crucial skill for physics students to master. By following the step-by-step process outlined in this guide, you can accurately calculate the total energy contained in a given number of photons, using the wavelength or frequency as the starting point. Remember to always double-check your work and refer to the provided formulas, examples, and data points to ensure your calculations are correct.
References
- https://sciencing.com/figure-energy-one-mole-photon-8664413.html
- https://www.vaia.com/en-us/textbooks/chemistry/chemistry-structure-and-properties-1-edition/chapter-3/problem-46-how-much-energy-is-contained-in-1-mol-of-each-beg/
- https://byjus.com/question-answer/calculate-energy-of-one-mole-of-photons-of-radiation-whose-frequency-as-5-times-10-1/

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.