Valence electrons are the key to understanding chemical bonding, reactivity, and stability of elements. They dictate an atom’s behavior in chemical reactions and determine its bonding capacity. By grasping the concept of valence electrons, we can unravel the intricate world of chemistry and the formation of compounds.
Periodic Table Method
The number of valence electrons can be easily determined using the periodic table method. This method works for the main group elements (s-block and p-block elements) on the periodic table.
S-Block Elements
For s-block elements, the number of valence electrons is equal to the group number on the periodic table. For example:
- Group 1 (alkali metals): 1 valence electron
- Group 2 (alkaline earth metals): 2 valence electrons
P-Block Elements
For p-block elements, the number of valence electrons is equal to the group number minus 10. For example:
- Group 13 (boron group): 3 valence electrons (13 – 10 = 3)
- Group 14 (carbon group): 4 valence electrons (14 – 10 = 4)
This method makes the valence electrons of the main group elements directly determinable.
Electronic Configuration Method
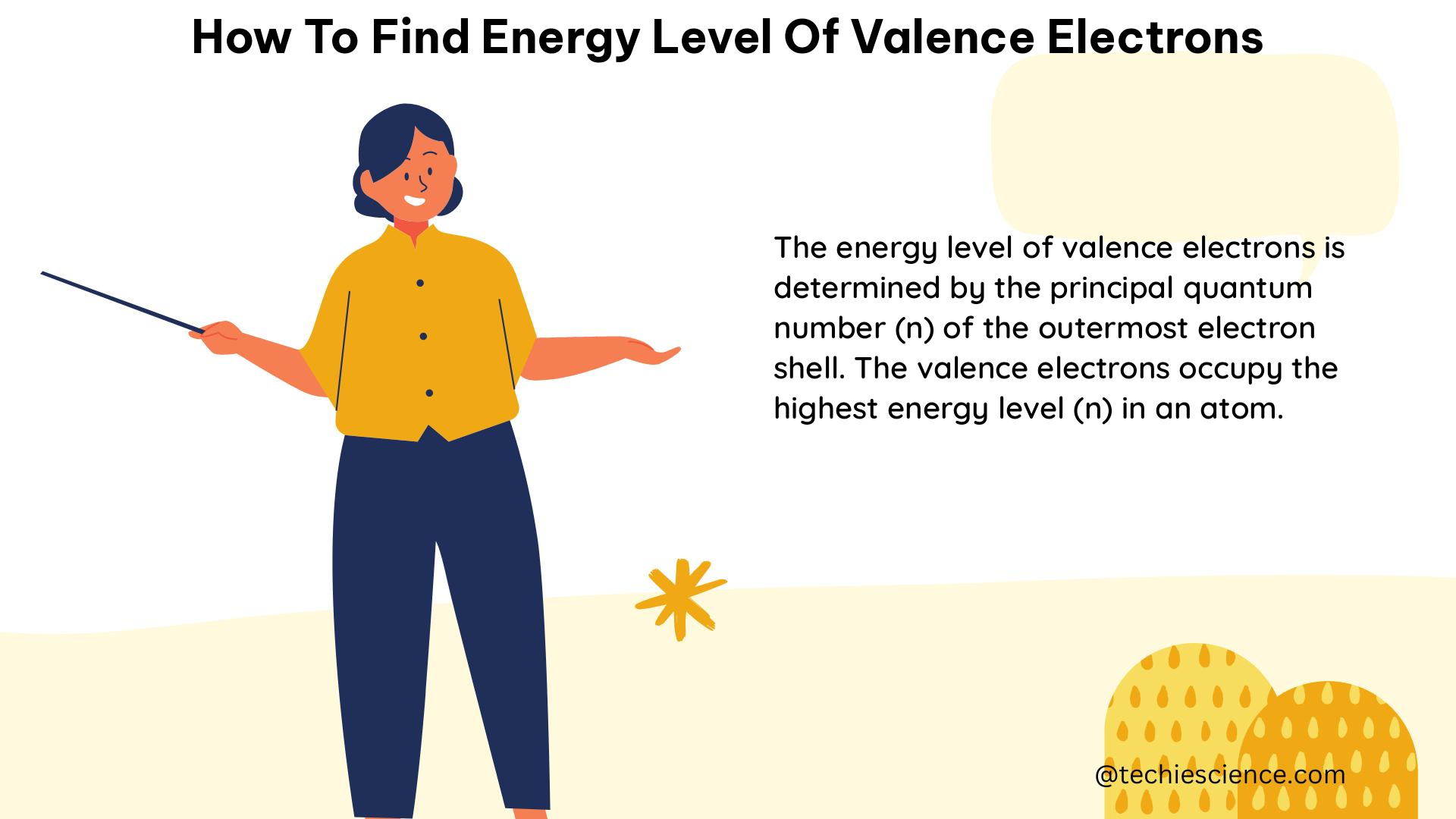
The electronic configuration method involves looking at the highest energy level electrons in the electron configuration of an element. The valence electrons are the electrons located in the outermost energy level of an atom.
Example: Copper (Cu)
The electron configuration of copper is: [Ar] 4s^1 3d^10
In this configuration, the 4th energy level is the highest energy level, and it has one valence electron.
Transition Metals
Transition metals have more complex electron configurations, and the d-subshell electrons in some elements participate in bonding despite not being at the highest energy level.
For example, in the electron configuration of chromium (Cr), the valence electrons are the 4s^1 3d^5 electrons, even though the 4s electrons are at a higher energy level than the 3d electrons.
Valence Electron Characteristics
Valence electrons are the outermost shell electrons located in an atom, which help in chemical bond formation. They are in the outermost part of the atom and are the least tightly held by the nucleus. Hence, they become available for sharing in bonding and various chemical reactions.
When two atoms interact, the electrons in the outermost shells are the first ones to come into contact with each other and are the ones that determine how an atom will react in a chemical reaction.
Valence electrons play a crucial role in:
– Chemical bonding
– Reactivity
– Electronegativity
– Stability of elements
Valence Electron Configurations
The valence electron configuration of an element can be represented using the following notation:
ns^x np^y
Where:
– n is the principal quantum number of the valence shell
– s and p represent the s and p subshells, respectively
– x and y are the number of electrons in the s and p subshells, respectively
For example:
– Sodium (Na): 3s^1
– Oxygen (O): 2s^2 2p^4
– Bromine (Br): 4s^2 4p^5
Valence Electron Diagrams
Valence electron diagrams, also known as Lewis dot structures, are a visual representation of the valence electrons in an atom. These diagrams show the arrangement of valence electrons around the nucleus of an atom.
In a valence electron diagram, the element symbol is surrounded by dots, with each dot representing a valence electron. The number of dots corresponds to the number of valence electrons in the atom.
For example, the valence electron diagram for sodium (Na) would be:
Na•
And the valence electron diagram for oxygen (O) would be:
O••
••
These diagrams are useful in understanding and predicting the chemical behavior of atoms, as well as in the formation of chemical bonds.
Valence Electron Calculations
The number of valence electrons can be calculated using the following formula:
Number of valence electrons = Group number (for main group elements)
For transition metals and other elements with complex electron configurations, the valence electrons can be determined by examining the highest energy level electrons in the electron configuration.
Valence Electron Examples
Let’s look at some examples to solidify our understanding of valence electrons:
- Magnesium (Mg):
- Atomic number: 12
- Group number: 2 (alkaline earth metal)
-
Number of valence electrons: 2
-
Chlorine (Cl):
- Atomic number: 17
- Group number: 17 (halogen)
-
Number of valence electrons: 17 – 10 = 7
-
Chromium (Cr):
- Atomic number: 24
- Electron configuration: [Ar] 4s^1 3d^5
-
Number of valence electrons: 6 (4s^1 3d^5)
-
Xenon (Xe):
- Atomic number: 54
- Group number: 18 (noble gas)
- Number of valence electrons: 8
By understanding the concepts of valence electrons and the methods to determine their energy levels, you can gain a deeper insight into the behavior of elements and the formation of chemical bonds.
Reference:

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.