In the captivating world of quantum mechanics, understanding the energy differences between atomic orbitals is a crucial step in unraveling the intricate dance of electrons within an atom. This comprehensive guide will equip you with the knowledge and tools necessary to navigate the complexities of this fundamental concept.
Unveiling the Energy Difference Formula
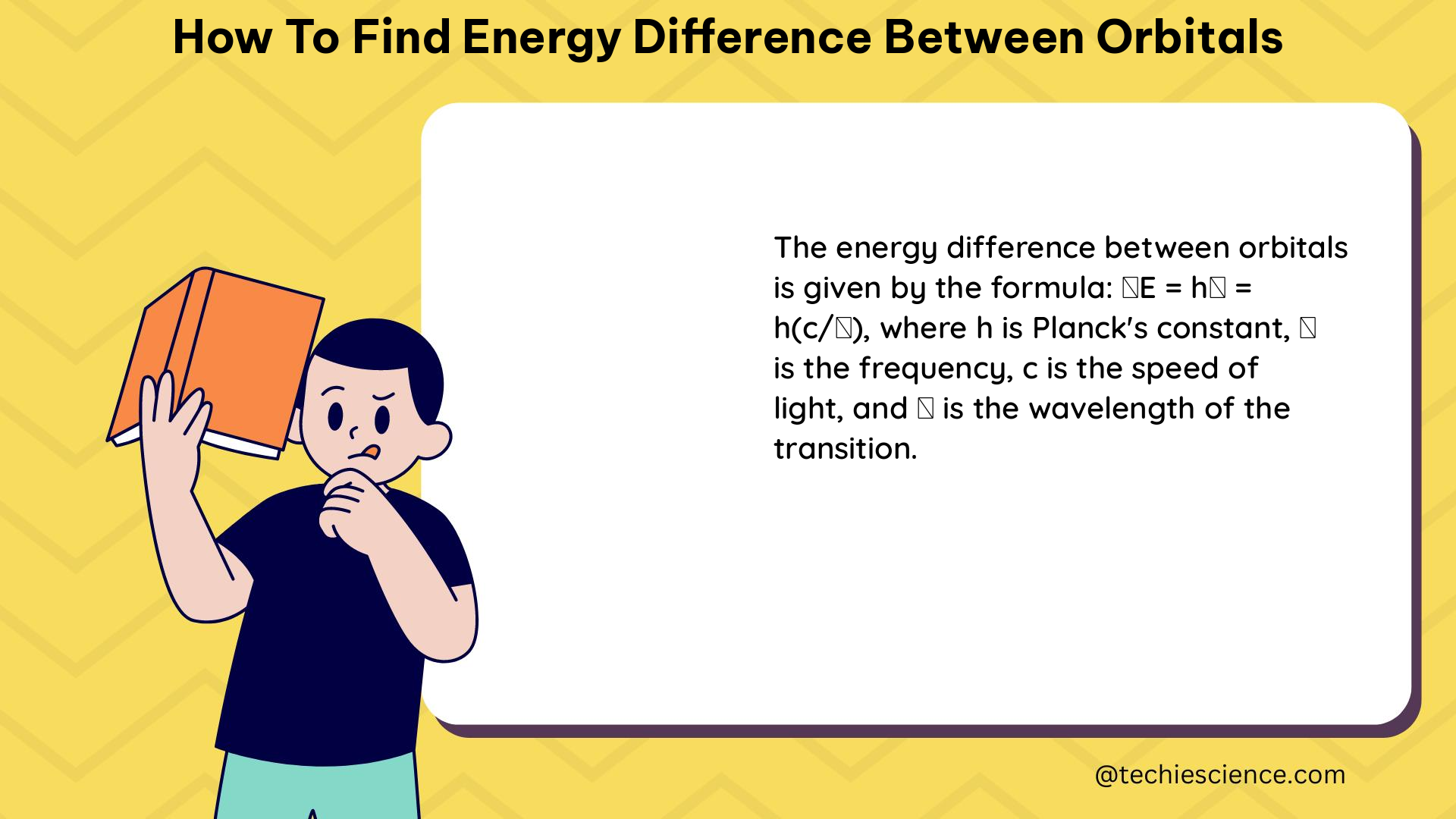
The energy difference between two orbitals can be calculated using the following formula:
ΔE = E(n2, l2) - E(n1, l1)
Where:
– ΔE represents the energy difference between the two orbitals
– E(n2, l2) is the energy of the higher-energy orbital
– E(n1, l1) is the energy of the lower-energy orbital
The principal quantum number n and the azimuthal quantum number l are the key players in determining the energy of an orbital. Let’s dive deeper into the role of these quantum numbers.
The Influence of Quantum Numbers
The energy of an orbital is directly influenced by both the principal quantum number n and the azimuthal quantum number l. Here’s how they contribute to the energy of an orbital:
- Principal Quantum Number (n):
- The principal quantum number
nrepresents the energy level or shell of the electron within the atom. - As
nincreases, the energy of the orbital increases, with the 1s orbital having the lowest energy and the 7s orbital having the highest energy. -
The energy of an orbital is inversely proportional to the square of the principal quantum number, i.e.,
E ∝ 1/n^2. -
Azimuthal Quantum Number (l):
- The azimuthal quantum number
lrepresents the angular momentum or the shape of the orbital. - For a given value of
n, the energy of the orbitals decreases aslincreases, with the s orbitals (l = 0) having the highest energy and the d orbitals (l = 2) having the lowest energy within the same energy level. - The energy of an orbital is inversely proportional to the sum of the principal quantum number
nand the azimuthal quantum numberl, i.e.,E ∝ 1/(n + l).
By understanding the relationship between the quantum numbers and the energy of an orbital, you can effectively calculate the energy difference between any two orbitals.
Effective Nuclear Charge (Zeff) and Shielding
In multi-electron atoms, the energy of an electron in a given orbital is not only influenced by the principal and azimuthal quantum numbers but also by the effective nuclear charge (Zeff) and the shielding effect.
- Effective Nuclear Charge (Zeff):
- The effective nuclear charge (Zeff) is the net positive charge experienced by the outer shell electrons in a multi-electron atom.
- Zeff is less than the actual nuclear charge (Z) due to the shielding effect of the inner shell electrons.
- As the atomic number (Z) increases, the value of Zeff also increases, leading to a higher attractive force between the nucleus and the outer shell electrons.
-
This increased attractive force results in a decrease in the energy of the outer shell electrons, making them more tightly bound to the nucleus.
-
Shielding Effect:
- The presence of inner shell electrons in a multi-electron atom shields the outer shell electrons from the full positive charge of the nucleus.
- This shielding effect reduces the attractive force between the nucleus and the outer shell electrons, leading to a decrease in the energy of the outer shell electrons.
- The degree of shielding depends on the number and configuration of the inner shell electrons, with the s and p orbitals providing more shielding than the d and f orbitals.
Understanding the concepts of effective nuclear charge and shielding is crucial in accurately calculating the energy differences between orbitals in multi-electron atoms.
Practical Examples and Numerical Problems
Let’s apply the knowledge we’ve gained to solve some practical examples and numerical problems related to finding the energy difference between orbitals.
Example 1: Hydrogen Atom
Consider the energy levels of a hydrogen atom:
– The energy of the 1s orbital is -13.6 eV.
– The energy of the 2s orbital is -3.4 eV.
To find the energy difference between the 2s and 1s orbitals, we can use the formula:
ΔE = E(n2, l2) - E(n1, l1)
ΔE = -3.4 eV - (-13.6 eV) = 10.2 eV
Therefore, the energy difference between the 2s and 1s orbitals in a hydrogen atom is 10.2 eV.
Example 2: Lithium Atom
In a lithium atom (Z = 3), the energy of the 2s orbital is -5.4 eV, and the energy of the 2p orbital is -5.1 eV.
To find the energy difference between the 2s and 2p orbitals, we can use the formula:
ΔE = E(n2, l2) - E(n1, l1)
ΔE = -5.1 eV - (-5.4 eV) = 0.3 eV
Therefore, the energy difference between the 2s and 2p orbitals in a lithium atom is 0.3 eV.
Numerical Problem 1
Calculate the energy difference between the 3d and 4s orbitals in a calcium atom (Z = 20).
Given:
– Energy of the 3d orbital: -11.2 eV
– Energy of the 4s orbital: -10.0 eV
Solution:
ΔE = E(n2, l2) - E(n1, l1)
ΔE = -10.0 eV - (-11.2 eV) = 1.2 eV
Therefore, the energy difference between the 3d and 4s orbitals in a calcium atom is 1.2 eV.
Numerical Problem 2
Find the energy difference between the 4p and 5s orbitals in a potassium atom (Z = 19).
Given:
– Energy of the 4p orbital: -4.34 eV
– Energy of the 5s orbital: -3.89 eV
Solution:
ΔE = E(n2, l2) - E(n1, l1)
ΔE = -3.89 eV - (-4.34 eV) = 0.45 eV
Therefore, the energy difference between the 4p and 5s orbitals in a potassium atom is 0.45 eV.
These examples and numerical problems demonstrate the practical application of the energy difference formula and the influence of quantum numbers, effective nuclear charge, and shielding on the energy of orbitals.
Conclusion
Mastering the art of finding the energy difference between orbitals is a crucial step in understanding the behavior of electrons within an atom. By familiarizing yourself with the energy difference formula, the role of quantum numbers, and the concepts of effective nuclear charge and shielding, you can confidently tackle a wide range of problems related to atomic structure and energy levels.
Remember, the journey of learning never ends, and there’s always more to explore in the captivating world of quantum mechanics. Keep practicing, exploring, and expanding your knowledge to become a true master of this fascinating field.
References
- Quantum Mechanics and Atomic Orbitals
- Energy of Orbitals
- How to Calculate the Energy Difference between Orbitals

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.