The activation energy of a chemical reaction is a crucial parameter that determines the rate at which the reaction occurs. By understanding the relationship between the rate constant, temperature, and activation energy, you can accurately determine the activation energy of a reaction. This comprehensive guide will walk you through the two main methods for finding the activation energy: the graphical method and the algebraic method.
Arrhenius Equation: The Foundation
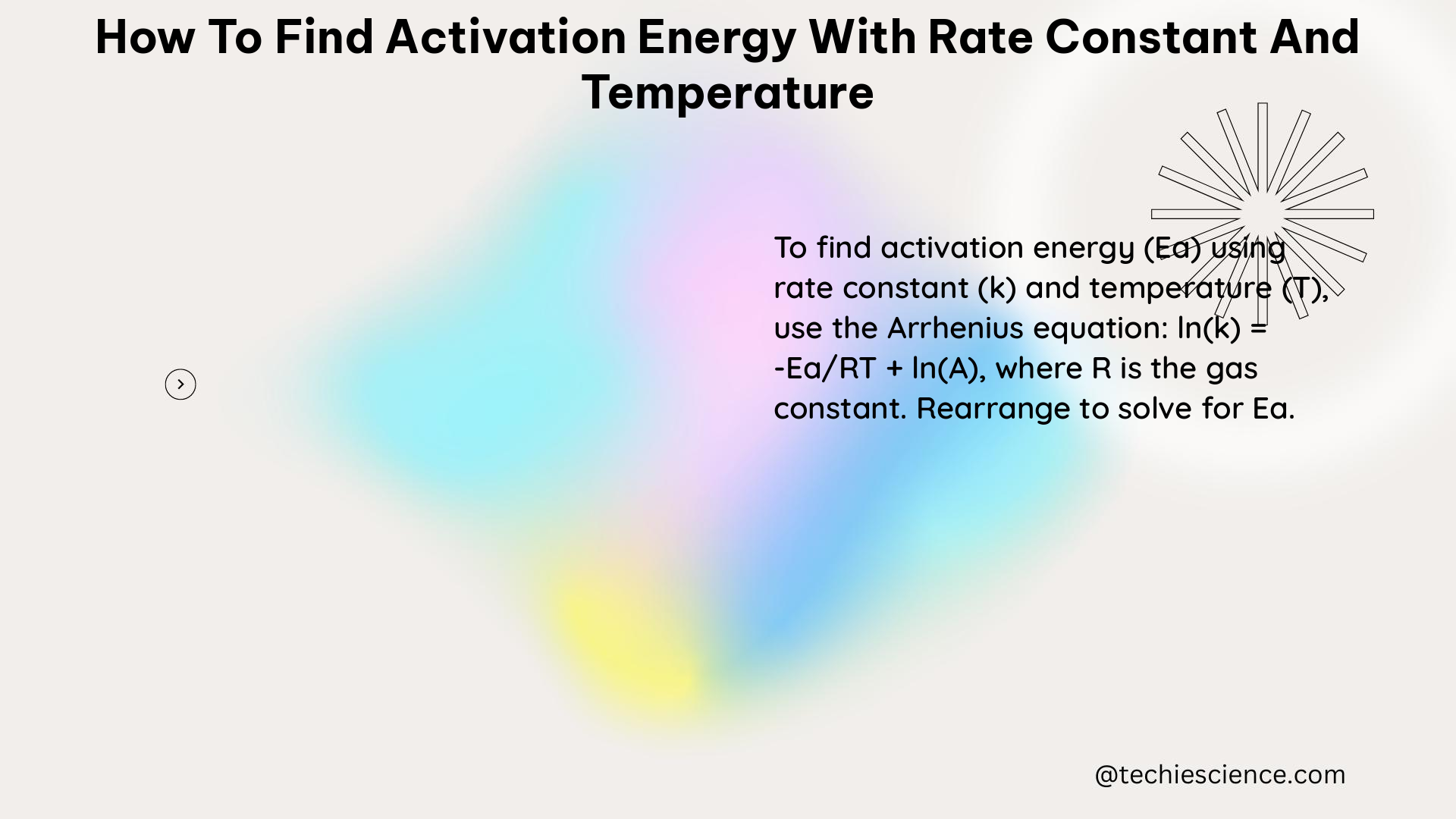
The Arrhenius equation is the fundamental relationship that connects the rate constant (k), activation energy (E_a), gas constant (R), and temperature (T):
k = A * e^(-E_a/RT)
Where:
– k is the rate constant
– A is the pre-exponential factor, also known as the frequency factor
– E_a is the activation energy
– R is the gas constant (8.314 J/mol·K)
– T is the absolute temperature in Kelvin (K)
This equation forms the basis for both the graphical and algebraic methods of determining the activation energy.
Graphical Method
The graphical method involves plotting the natural logarithm of the rate constant (ln k) against the reciprocal of the absolute temperature (1/T). The steps are as follows:
- Convert Temperatures to Kelvin: Ensure that all temperature values are in Kelvin (K) before proceeding.
- Calculate ln k: For each rate constant and temperature pair, calculate the natural logarithm of the rate constant (ln k).
- Plot ln k vs. 1/T: Create a scatter plot with ln k on the y-axis and 1/T on the x-axis.
- Determine the Slope: The slope of the linear graph is equal to -E_a/R.
- Calculate E_a: Use the slope of the line to find the activation energy:
E_a = -slope * R
Here’s an example using the following data:
| Temperature (K) | Rate Constant, k (s^-1) |
|---|---|
| 298 | 1.74 × 10^-5 |
| 308 | 6.61 × 10^-5 |
| 318 | 2.51 × 10^-4 |
| 328 | 7.59 × 10^-4 |
| 338 | 2.40 × 10^-3 |
Plotting ln k vs. 1/T and finding the slope, we get:
E_a = -(-8e-05) * 8.314 = 103 kJ/mol
Algebraic Method
The algebraic method uses two rate constants and their corresponding temperatures to solve for the activation energy. The steps are as follows:
- Use Two Rate Constants and Temperatures: Given two rate constants (k_1 and k_2) at two different temperatures (T_1 and T_2), use the Arrhenius equation to express each rate constant:
ln k_1 = ln A - E_a/(R*T_1)
ln k_2 = ln A - E_a/(R*T_2)
- Eliminate A: Subtract the first equation from the second to eliminate the frequency factor A:
ln k_2 - ln k_1 = E_a/R * (1/T_1 - 1/T_2)
- Solve for E_a: Rearrange the equation to solve for the activation energy:
E_a = R * ln(k_2/k_1) / (1/T_1 - 1/T_2)
Here’s an example using the following data:
– k_1 = 5.4 × 10^-4 M^-1·s^-1 at T_1 = 599 K
– k_2 = 2.8 × 10^-2 M^-1·s^-1 at T_2 = 683 K
Plugging in the values, we get:
E_a = R * ln(k_2/k_1) / (1/T_1 - 1/T_2)
= 8.314 J/mol·K * ln(2.8e-2/5.4e-4) / (1/599 K - 1/683 K)
= 1.60 × 10^5 J/mol
Key Considerations
- The Arrhenius equation is the foundation for both the graphical and algebraic methods.
- The graphical method involves plotting ln k vs. 1/T and using the slope to calculate the activation energy.
- The algebraic method uses two rate constants and their corresponding temperatures to solve for the activation energy.
- Ensure that all temperature values are in Kelvin (K) and that the units of the rate constants and activation energy are consistent.
- The activation energy represents the minimum energy required for a reaction to occur, and it is a crucial parameter in understanding and predicting reaction kinetics.
References
- LibreTexts. (2019). 4.6: Activation Energy and Rate. https://chem.libretexts.org/Courses/Bellarmine_University/BU:Chem_104(Christianson)/Phase_2:_Understanding_Chemical_Reactions/4:_Kinetics:_How_Fast_Reactions_Go/4.6:_Activation_Energy_and_Rate
- YouTube. (2020). Calculate Activation Energy from Rate Constants and Temperatures (Slope). https://www.youtube.com/watch?v=E6L12eosb38
- Florida State University. Activation Energy. https://www.chem.fsu.edu/chemlab/chm1046course/activation.html
- ThoughtCo. (2019). How to Calculate Activation Energy. https://www.thoughtco.com/activation-energy-example-problem-609456
- Lumen Learning. Activation Energy and the Arrhenius Equation. https://courses.lumenlearning.com/suny-introductorychemistry/chapter/activation-energy-and-the-arrhenius-equation-2/

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.