The photoelectric effect is a fundamental phenomenon in quantum mechanics where electrons are emitted from a metal surface when light of sufficient energy strikes the surface. By understanding the principles of the photoelectric effect, we can determine the velocity of the emitted electrons, which has important applications in various fields of physics. This comprehensive guide will walk you through the step-by-step process of determining the velocity of electrons using the photoelectric effect.
Understanding the Photoelectric Effect
The photoelectric effect occurs when a photon of light with sufficient energy strikes the surface of a metal, causing an electron to be ejected from the metal. The key factors that govern the photoelectric effect are:
-
Incident Light Energy (Ep): The energy of the incident photon, which is determined by the frequency of the light using the equation
Ep = hf, wherehis Planck’s constant (6.63 × 10^-34 J·s) andfis the frequency of the light. -
Work Function (Φ): The minimum energy required to remove an electron from the metal, which is a characteristic property of the metal.
-
Kinetic Energy (K): The energy of the emitted electron, which is the difference between the incident photon energy and the work function, given by the equation
K = Ep - Φ.
Determining the Velocity of Emitted Electrons
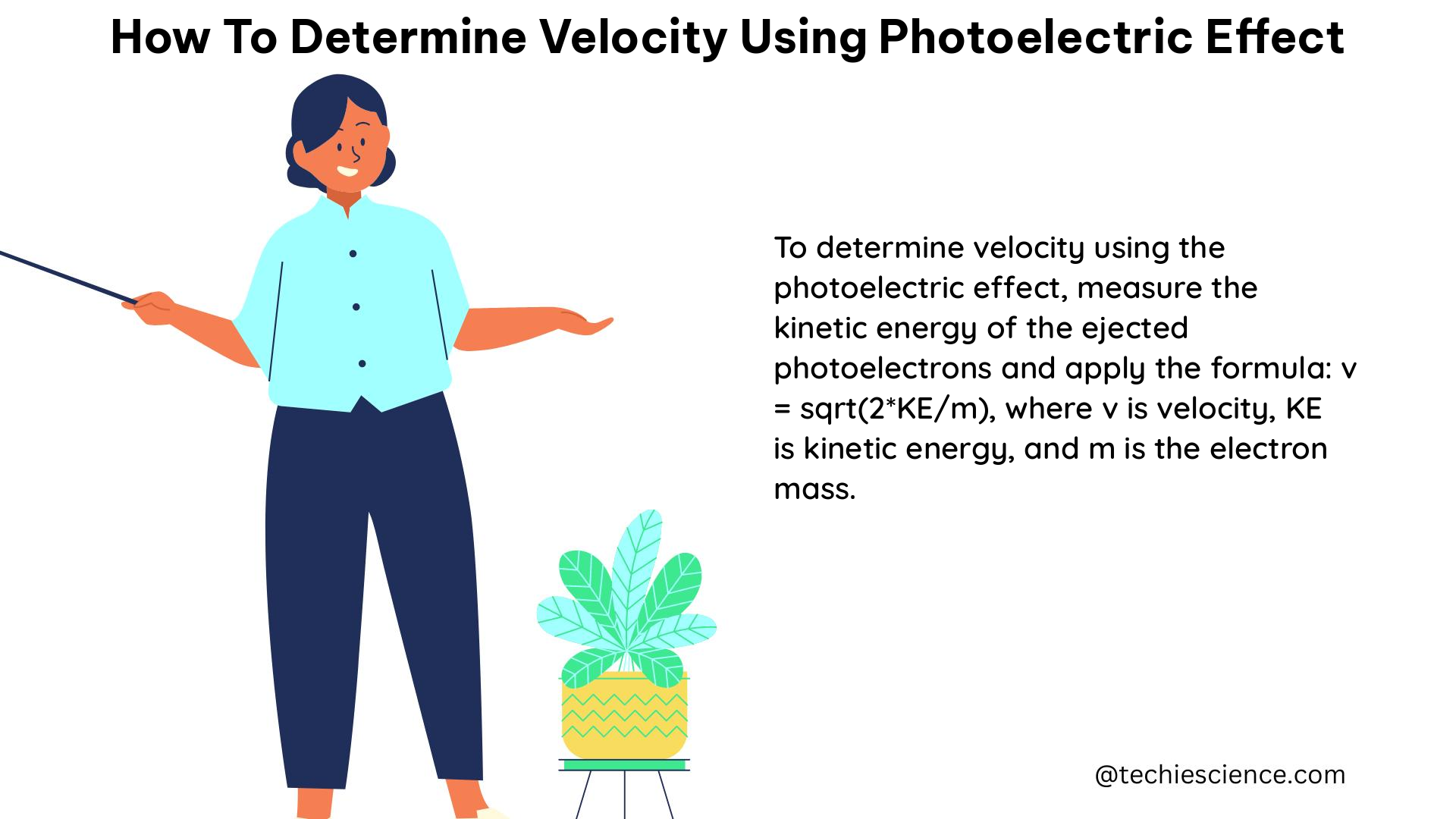
To determine the velocity of the emitted electrons in a photoelectric effect experiment, follow these steps:
- Calculate the Energy of the Incident Light:
- Use the equation
Ep = hfto calculate the energy of the incident photon, wherehis Planck’s constant andfis the frequency of the light. -
Example: If the frequency of the incident light is 2.1 × 10^15 Hz, the energy of the incident photon would be:
Ep = hf
Ep = (6.63 × 10^-34 J·s) × (2.1 × 10^15 Hz)
Ep = 1.392 × 10^-18 J -
Calculate the Kinetic Energy of the Emitted Electrons:
- Use the equation
K = Ep - Φto calculate the kinetic energy of the emitted electrons, whereEpis the energy of the incident photon andΦis the work function of the metal. -
Example: If the work function of the metal is 4.7 eV, the kinetic energy of the emitted electrons would be:
K = Ep - Φ
K = 1.392 × 10^-18 J - (4.7 eV × 1.6 × 10^-19 J/eV)
K = 1.392 × 10^-18 J - 7.52 × 10^-19 J
K = 6.402 × 10^-19 J -
Convert the Kinetic Energy to Joules:
- The kinetic energy calculated in step 2 is in electron volts (eV). Convert it to Joules by multiplying by the conversion factor 1.6 × 10^-19 J/eV.
-
Example:
K = 6.402 × 10^-19 J -
Calculate the Velocity of the Emitted Electrons:
- Use the equation
v = √(2K/m)to calculate the velocity of the emitted electrons, whereKis the kinetic energy in Joules andmis the mass of an electron (9.11 × 10^-31 kg). - Example:
v = √[(2 × 6.402 × 10^-19 J) / (9.11 × 10^-31 kg)] - Solving the equation, the velocity of the emitted electrons is
v = 1.19 × 10^6 m/s.
By following these steps, you can determine the velocity of the electrons emitted in a photoelectric effect experiment, given the frequency of the incident light and the work function of the metal.
Additional Considerations and Examples
-
Dependence on Incident Light Frequency: The velocity of the emitted electrons is directly proportional to the square root of the incident light frequency. As the frequency of the light increases, the velocity of the emitted electrons also increases.
-
Dependence on Work Function: The velocity of the emitted electrons is inversely proportional to the square root of the work function of the metal. As the work function increases, the velocity of the emitted electrons decreases.
-
Example Calculation: Let’s consider another example where the incident light has a frequency of 3.0 × 10^15 Hz and the work function of the metal is 5.1 eV.
-
Calculate the energy of the incident light:
Ep = hf
Ep = (6.63 × 10^-34 J·s) × (3.0 × 10^15 Hz)
Ep = 1.989 × 10^-18 J -
Calculate the kinetic energy of the emitted electrons:
K = Ep - Φ
K = 1.989 × 10^-18 J - (5.1 eV × 1.6 × 10^-19 J/eV)
K = 1.989 × 10^-18 J - 8.16 × 10^-19 J
K = 1.173 × 10^-18 J -
Calculate the velocity of the emitted electrons:
v = √(2K/m)
v = √[(2 × 1.173 × 10^-18 J) / (9.11 × 10^-31 kg)]
v = 1.43 × 10^6 m/s
In this example, the velocity of the emitted electrons is 1.43 × 10^6 m/s.
By understanding the principles of the photoelectric effect and the equations involved, you can accurately determine the velocity of the emitted electrons in various scenarios. This knowledge is crucial for understanding the behavior of electrons in quantum mechanics and has applications in fields such as electronics, spectroscopy, and particle physics.
References:
- The Photoelectric Effect | Physics – Lumen Learning: https://courses.lumenlearning.com/suny-physics/chapter/29-2-the-photoelectric-effect/
- Photoelectric effect find velocity? – Physics Forums: https://www.physicsforums.com/threads/photoelectric-effect-find-velocity.893046/
- Finding the Average Velocity of Electrons Emitted in a Photoelectric Experiment with Given Incident Light Frequency, Material Work Function – Explanation: https://study.com/skill/learn/finding-the-average-velocity-of-electrons-emitted-in-a-photoelectric-experiment-with-given-incident-light-frequency-material-work-function-explanation.html

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.