Calculating the chemical energy released in an alkaline battery involves understanding the underlying electrochemical reactions, the battery’s capacity, voltage, and efficiency. This comprehensive guide will provide you with the necessary formulas, examples, and technical details to accurately determine the chemical energy output of an alkaline battery.
Understanding Alkaline Battery Chemistry
Alkaline batteries, such as the common AA or AAA types, operate based on the redox reaction between zinc (Zn) and manganese dioxide (MnO2). The overall reaction can be represented as:
Anode reaction: Zn + 2OH- → ZnO + H2O + 2e-
Cathode reaction: 2MnO2 + H2O + 2e- → 2MnOOH
Overall reaction: Zn + 2MnO2 → ZnO + 2MnOOH
This electrochemical reaction generates a nominal voltage of around 1.5 volts, which is the standard voltage for alkaline batteries.
Calculating the Chemical Energy
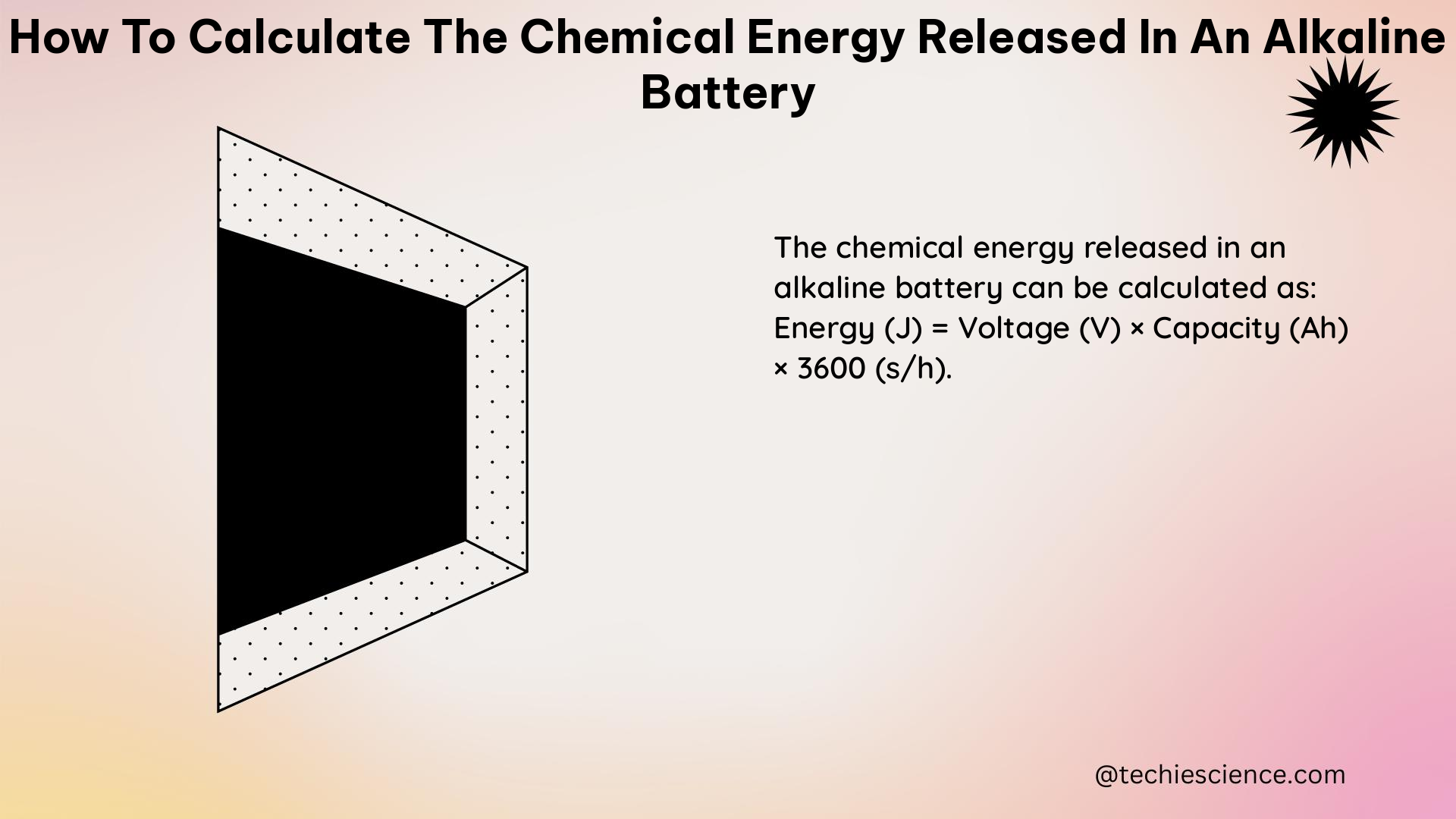
The chemical energy (E) released by an alkaline battery can be calculated using the following formula:
E = V × C × e
Where:
– E is the chemical energy in watt-hours (Wh)
– V is the voltage of the battery in volts (V)
– C is the capacity of the battery in ampere-hours (Ah)
– e is the efficiency of the battery, typically around 0.7 for alkaline batteries
Let’s consider an example:
Suppose we have an AA alkaline battery with the following specifications:
– Voltage (V) = 1.5 V
– Capacity (C) = 2500 mAh = 2.5 Ah
– Efficiency (e) = 0.7 (70%)
Plugging these values into the formula:
E = 1.5 V × 2.5 Ah × 0.7 = 2.625 Wh
This means that the theoretical maximum chemical energy that can be released by this AA alkaline battery is 2.625 watt-hours.
Factors Affecting Chemical Energy Release
The actual chemical energy released by an alkaline battery can be influenced by several factors:
-
Discharge Rate: The discharge rate, or the current drawn from the battery, can significantly impact its capacity and efficiency. Higher discharge rates can lead to lower capacity and efficiency.
-
Temperature: The performance of alkaline batteries is affected by temperature. Lower temperatures can result in reduced capacity and efficiency.
-
Battery Age: As alkaline batteries age, their internal resistance increases, leading to a decrease in capacity and efficiency.
To account for these factors, more complex models can be used, which take into consideration the battery’s internal resistance, temperature coefficients, and other parameters. However, these models can be more challenging to implement and may require specialized knowledge and software.
Advanced Calculations and Considerations
For a more comprehensive understanding of alkaline battery energy calculations, consider the following additional factors and techniques:
Internal Resistance and Voltage Drop
The internal resistance of an alkaline battery can cause a voltage drop under load, reducing the effective voltage and, consequently, the available chemical energy. This can be calculated using Ohm’s law:
V_load = V_open – I × R_internal
Where:
– V_load is the voltage under load
– V_open is the open-circuit voltage
– I is the discharge current
– R_internal is the internal resistance of the battery
Peukert’s Law
Peukert’s law describes the relationship between a battery’s capacity and its discharge rate. It can be used to estimate the available capacity at different discharge rates:
C_actual = C_rated × (I_rated / I_actual)^(1/n)
Where:
– C_actual is the actual capacity at the given discharge rate
– C_rated is the rated capacity of the battery
– I_rated is the rated discharge current
– I_actual is the actual discharge current
– n is the Peukert exponent, typically between 1.1 and 1.3 for alkaline batteries
Ragone Plot
The Ragone plot is a graphical representation of a battery’s power density (W/kg) versus its energy density (Wh/kg). This plot can help visualize the trade-off between power and energy for different battery technologies, including alkaline batteries.
Thermal Considerations
The heat generated during the discharge of an alkaline battery can affect its performance and safety. Factors such as the battery’s internal resistance, discharge rate, and ambient temperature can influence the thermal behavior and should be considered in advanced calculations.
By understanding these advanced concepts and techniques, you can gain a deeper insight into the chemical energy release dynamics of alkaline batteries and optimize their performance for your specific applications.
Conclusion
Calculating the chemical energy released in an alkaline battery involves a straightforward formula that considers the battery’s voltage, capacity, and efficiency. However, to accurately predict the energy output, it’s essential to account for factors such as discharge rate, temperature, and battery age. By incorporating these considerations and exploring advanced techniques like internal resistance analysis, Peukert’s law, and thermal management, you can develop a comprehensive understanding of alkaline battery energy dynamics and optimize their usage in various applications.
References:
- Alkaline Manganese Dioxide – Energizer. https://data.energizer.com/pdfs/alkaline_appman.pdf
- Battery Runtime Calculator. https://www.translatorscafe.com/unit-converter/en-US/calculator/battery-runtime/
- How Do Alkaline Batteries Work – BYJU’S. https://byjus.com/chemistry/how-do-alkaline-batteries-work/
- Battery University. https://batteryuniversity.com/learn/article/alkaline_battery
- Peukert’s Law Explained. https://www.mpoweruk.com/peukert.htm
- Ragone Plot Explained. https://www.mpoweruk.com/performance.htm

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.