The energy value of food is a crucial factor in understanding the nutritional content and caloric intake of our diets. To accurately calculate the energy value of food, various methods and systems have been developed, each with its own advantages and considerations. In this comprehensive guide, we will delve into the details of these methods, providing you with the necessary knowledge and tools to effectively calculate the energy value of food.
The Atwater System: Estimating Energy Value
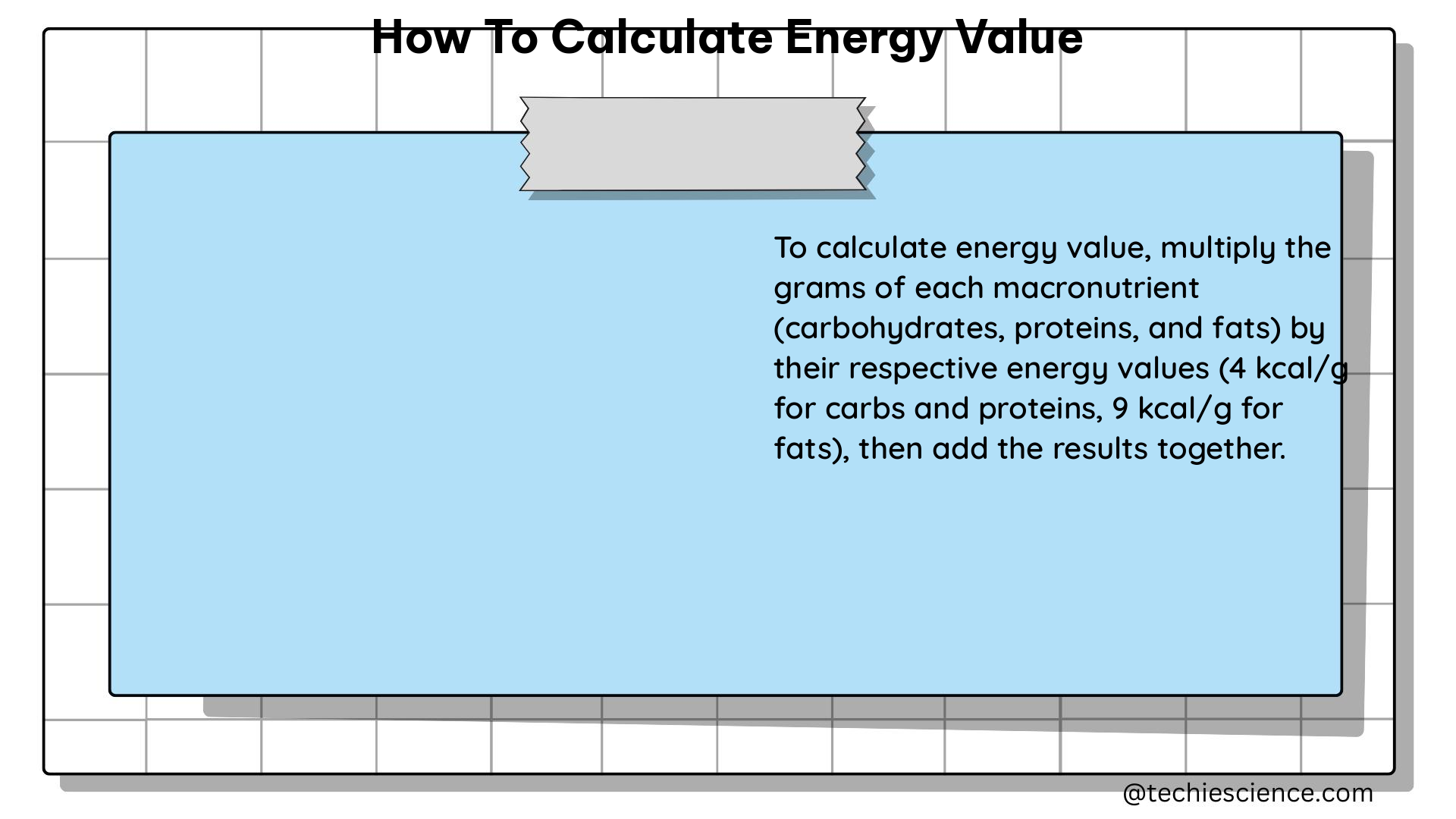
The Atwater system is the most commonly used method for calculating the energy value of food. This system estimates the energy value based on the amounts of protein, fat, and carbohydrates present in the food. The energy values assigned to these macronutrients are as follows:
- Protein: 4 kcal/g
- Fat: 9 kcal/g
- Carbohydrates: 4 kcal/g
To calculate the energy value using the Atwater system, you can follow these steps:
- Determine the amounts of protein, fat, and carbohydrates in the food (in grams).
- Multiply the amount of each macronutrient by its respective energy value.
- Add the energy values of the three macronutrients to obtain the total energy value of the food.
For example, let’s say a food item contains 10 g of protein, 5 g of fat, and 15 g of carbohydrates. The energy value calculation would be:
- Protein: 10 g × 4 kcal/g = 40 kcal
- Fat: 5 g × 9 kcal/g = 45 kcal
- Carbohydrates: 15 g × 4 kcal/g = 60 kcal
- Total energy value: 40 kcal + 45 kcal + 60 kcal = 145 kcal
It’s important to note that the Atwater system provides estimates, and the actual energy values may vary depending on the specific food and its composition.
Bomb Calorimetry: Measuring Combustible Energy
Bomb calorimetry is a more accurate method for determining the energy value of food. This technique involves burning a food sample in a sealed, pressurized chamber (a bomb calorimeter) and measuring the heat released during the combustion process. The heat released is directly proportional to the total combustible energy content of the food.
The steps involved in using a bomb calorimeter to calculate the energy value of food are as follows:
- Weigh the food sample and place it in the bomb calorimeter.
- Fill the bomb calorimeter with a known volume of water and measure its initial temperature.
- Ignite the food sample, causing it to burn completely.
- Measure the final temperature of the water in the bomb calorimeter.
- Calculate the heat absorbed by the water using the formula: q = m × c × ΔT, where:
- q is the heat absorbed (in joules)
- m is the mass of the water (in grams)
- c is the specific heat capacity of water (4.184 J/g°C)
- ΔT is the change in temperature (in °C)
- Divide the total heat absorbed by the mass of the food sample to obtain the energy value per gram of the food.
Bomb calorimetry is considered more accurate than the Atwater system, as it directly measures the total combustible energy content of the food. However, it is also more time-consuming and expensive to perform.
Accounting for Energy Lost During Digestion and Absorption
It’s important to note that not all the energy contained in food is available to the body. Some energy is lost during the processes of digestion and absorption. The net metabolizable energy (NME) system is a method that accounts for this energy loss by modifying the metabolizable energy (ME) values to consider the energy lost as heat from different substrates via heat of fermentation and obligatory thermogenesis.
The NME system uses the following formula to calculate the net metabolizable energy:
NME = ME – (Heat of fermentation + Obligatory thermogenesis)
Where:
– NME is the net metabolizable energy
– ME is the metabolizable energy (calculated using the Atwater system)
– Heat of fermentation is the energy lost as heat during the fermentation of undigested carbohydrates
– Obligatory thermogenesis is the energy lost as heat during the digestion and absorption of food
By incorporating these energy losses, the NME system provides a more accurate representation of the energy that is actually available to the body.
Laboratory Techniques for Calculating Energy Value
In a laboratory setting, the energy value of food can be determined using various techniques, such as bomb calorimetry and indirect calorimetry.
Bomb Calorimetry
As mentioned earlier, bomb calorimetry involves burning a food sample in a sealed, pressurized chamber and measuring the heat released during the combustion process. This method provides a direct measurement of the total combustible energy content of the food.
Indirect Calorimetry
Indirect calorimetry is another laboratory technique used to calculate the energy value of food. This method involves measuring the oxygen consumption and carbon dioxide production of an individual or animal after consuming the food. The energy value can then be estimated based on the respiratory exchange ratio (RER) and the energy equivalent of oxygen.
The general steps for calculating the energy value using indirect calorimetry are:
- Measure the oxygen consumption (VO2) and carbon dioxide production (VCO2) of the individual or animal after consuming the food.
- Calculate the RER using the formula: RER = VCO2 / VO2
- Determine the energy equivalent of oxygen based on the RER and the caloric values of the macronutrients.
- Calculate the energy value by multiplying the oxygen consumption by the energy equivalent of oxygen.
Both bomb calorimetry and indirect calorimetry provide more accurate measurements of the energy value of food compared to the Atwater system, but they require specialized equipment and expertise to perform.
Factors Affecting Energy Value Calculations
Several factors can influence the accuracy of energy value calculations, including:
- Food Composition: The specific composition of a food, including the proportions of protein, fat, and carbohydrates, can affect the energy value.
- Cooking and Processing: The way a food is prepared, such as cooking, can alter its energy value by changing the availability of nutrients and the digestibility of the food.
- Individual Differences: The energy value of a food may vary depending on the individual’s digestive system, metabolism, and other physiological factors.
- Measurement Errors: Inaccuracies in the measurement of food composition or the use of laboratory equipment can lead to errors in energy value calculations.
It’s important to consider these factors when calculating the energy value of food to ensure the most accurate and reliable results.
Conclusion
Calculating the energy value of food is a crucial step in understanding the nutritional content and caloric intake of our diets. The Atwater system, bomb calorimetry, and the net metabolizable energy (NME) system are the primary methods used to determine the energy value of food. Each method has its own advantages and considerations, and the choice of method will depend on the specific needs and resources available.
By understanding the principles and techniques behind these methods, you can effectively calculate the energy value of food and make informed decisions about your dietary intake. Remember to consider the various factors that can affect the accuracy of these calculations, and always strive for the most reliable and accurate results.
References
- ENERGY VALUE OF FOODS – USDA ARS
- An Easy Approach to Calculating Estimated Energy Requirements
- Science Behind Our Food
- CALCULATION OF THE ENERGY CONTENT OF FOODS
- Energy content in foods | Experiment – RSC Education
- Determination of the Energy Value of Foods by Bomb Calorimetry
- The effect of cooking and processing on the energy value of foods
- A review of methods for the determination of the energy value of food
- The energy cost of digestion and absorption of food in man
- The thermic effect of food and its importance for energy balance
- Energy Metabolism: Tissue Determinants and Regulation of Energy Balance
- The availability of energy from food and its measurement
- Energy requirements and physical activity levels

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.