Summary
Energy transfer is a fundamental concept in physics, and understanding how to calculate it is crucial for various applications, from thermodynamics to mechanical engineering. This comprehensive guide will provide you with a detailed understanding of the different formulas and methods used to calculate energy transferred, along with practical examples and numerical problems to help you master the topic.
Understanding the Basics of Energy Transfer
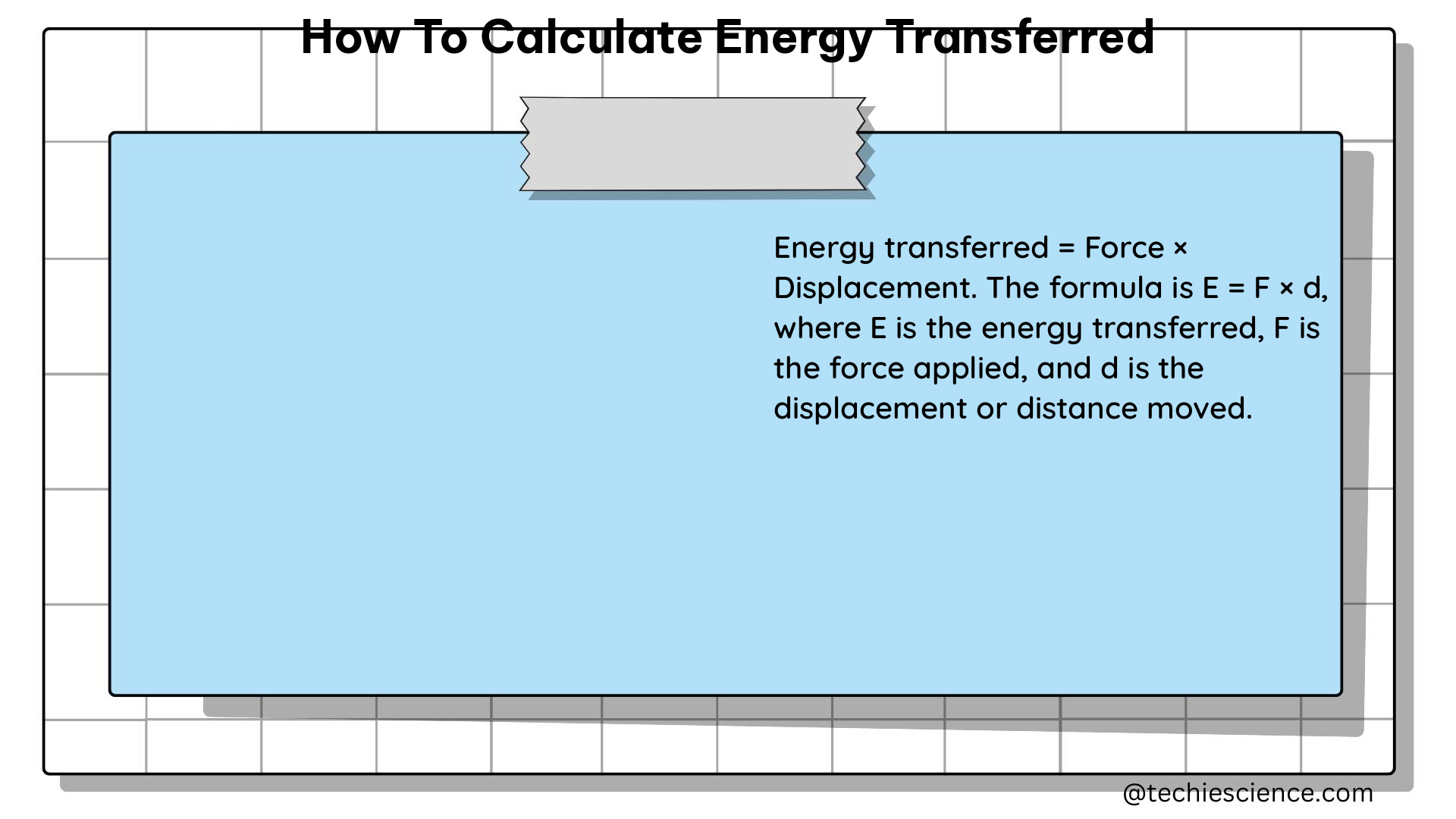
Energy transfer is the process by which energy is moved from one system or object to another. This can occur through various mechanisms, such as heat, work, or radiation. The amount of energy transferred can be quantified using various physical quantities, such as temperature, mass, and specific heat capacity.
Calculating Energy Transferred Using the Formula q = mcΔT
The most common formula used to calculate energy transferred is the formula q = mcΔT, where:
qis the energy transferred (in joules, J)mis the mass of the object (in kilograms, kg)cis the specific heat capacity of the object (in joules per kilogram per degree Celsius, J/kg°C)ΔTis the change in temperature (in degrees Celsius, °C)
Example 1: Calculating Energy Transferred in a Heating Process
Suppose we have a block of aluminum with a mass of 2 kg and a specific heat capacity of 900 J/kg°C. If we place this block in 1 liter (or 1 kg) of water at 25°C, and the final temperature becomes 30°C, we can calculate the energy transferred as follows:
q = mcΔT
q = (2 kg)(900 J/kg°C)(5°C)
q = 9000 J
Therefore, the energy transferred is 9000 joules.
Example 2: Calculating Energy Transferred in a Cooling Process
Consider a scenario where a hot object with a mass of 0.5 kg and a specific heat capacity of 840 J/kg°C cools down from 80°C to 25°C. To calculate the energy transferred, we can use the same formula:
q = mcΔT
q = (0.5 kg)(840 J/kg°C)(-55°C)
q = -23,100 J
The negative sign indicates that the energy was transferred from the hot object to the surroundings, resulting in a decrease in the object’s temperature.
Calculating Energy Transferred Using the Formula P = W/t
Another way to calculate energy transferred is by using the formula P = W/t, where:
Pis the power (in watts, W)Wis the work done (in joules, J)tis the time taken (in seconds, s)
This formula is particularly useful when you have information about the power and the time taken for the energy transfer to occur.
Example 3: Calculating Energy Transferred Using the Power Formula
Suppose a motor is used to lift a 50 kg object to a height of 2 meters in 10 seconds. To calculate the energy transferred, we can use the formula:
P = W/t
W = P × t
W = (F × d) / t
W = (m × g × h) / t
W = (50 kg × 9.8 m/s² × 2 m) / 10 s
W = 980 J
Therefore, the energy transferred in this process is 980 joules.
Calculating Energy Stored Gravitationally Using the Formula E = mgh
In addition to the formulas mentioned above, we can also use the formula E = mgh to calculate the energy stored gravitationally, where:
Eis the energy stored gravitationally (in joules, J)mis the mass of the object (in kilograms, kg)gis the acceleration due to gravity (in meters per second squared, m/s²)his the height (in meters, m)
Example 4: Calculating Energy Stored Gravitationally
Consider a trolley with a mass of 10 kg that is lifted to a height of 2 meters. We can calculate the energy stored gravitationally as follows:
E = mgh
E = (10 kg)(9.8 m/s²)(2 m)
E = 196 J
Therefore, the energy stored gravitationally in this scenario is 196 joules.
Advanced Concepts and Numerical Problems
In addition to the examples provided, there are several advanced concepts and numerical problems that can help you deepen your understanding of energy transfer calculations. These include:
-
Thermal Equilibrium and Heat Transfer: Explore the concept of thermal equilibrium and how it relates to the transfer of heat between objects with different temperatures.
-
Latent Heat and Phase Changes: Understand the role of latent heat in energy transfer during phase changes, such as melting, boiling, and condensation.
-
Conduction, Convection, and Radiation: Investigate the different modes of heat transfer and how they affect the calculation of energy transferred.
-
Efficiency and Energy Losses: Analyze the concept of efficiency and how it impacts the calculation of energy transferred, considering factors such as friction and other energy losses.
-
Numerical Problems with Multiple Stages: Solve complex problems that involve multiple steps or stages of energy transfer, requiring the application of various formulas and principles.
By exploring these advanced topics and solving numerical problems, you will develop a comprehensive understanding of energy transfer calculations and be able to apply them to a wide range of physics problems.
Conclusion
Calculating energy transferred is a fundamental skill in physics, with applications in various fields. This comprehensive guide has provided you with the necessary tools and knowledge to master the topic, including the use of the q = mcΔT, P = W/t, and E = mgh formulas, as well as practical examples and advanced concepts. By understanding these principles and applying them to numerical problems, you will be well-equipped to tackle energy transfer challenges in your studies and future endeavors.
References
- Calculating Energy Transfer part 1 – YouTube: https://www.youtube.com/watch?v=GQyGsk45qMw
- energy_transfer [Data Lab] – msuperl.org: https://www.msuperl.org/wikis/datalab/doku.php?id=energy_transfer
- Calculating Energy Transfer – YouTube: https://www.youtube.com/watch?v=CuqJBGHsT4o
- Measuring energy transfers | IOPSpark – Institute of Physics: https://spark.iop.org/collections/measuring-energy-transfers
- 13.2: Specific Heat – Physics LibreTexts: https://phys.libretexts.org/Bookshelves/University_Physics/Physics_(Boundless)/13%3A_Heat_and_Heat_Transfer/13.2%3A_Specific_Heat

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.