Calculating the density of substances at different temperatures is a crucial skill in various fields, including physics, chemistry, and engineering. This comprehensive guide will walk you through the step-by-step process of determining the density of both liquids and gases at varying temperatures, providing you with the necessary formulas, coefficients, and examples to master this concept.
Understanding the Relationship between Temperature and Density
The density of a substance is defined as the mass per unit volume, expressed in kilograms per cubic meter (kg/m³). The relationship between temperature and density is governed by the thermal expansion of the material. As the temperature of a substance changes, its volume also changes, which in turn affects its density.
For liquids, the density typically decreases as the temperature increases, due to the expansion of the material. Conversely, for gases, the density typically decreases as the temperature increases, as the gas molecules become more energetic and spread out.
Calculating Density of Liquids at Different Temperatures
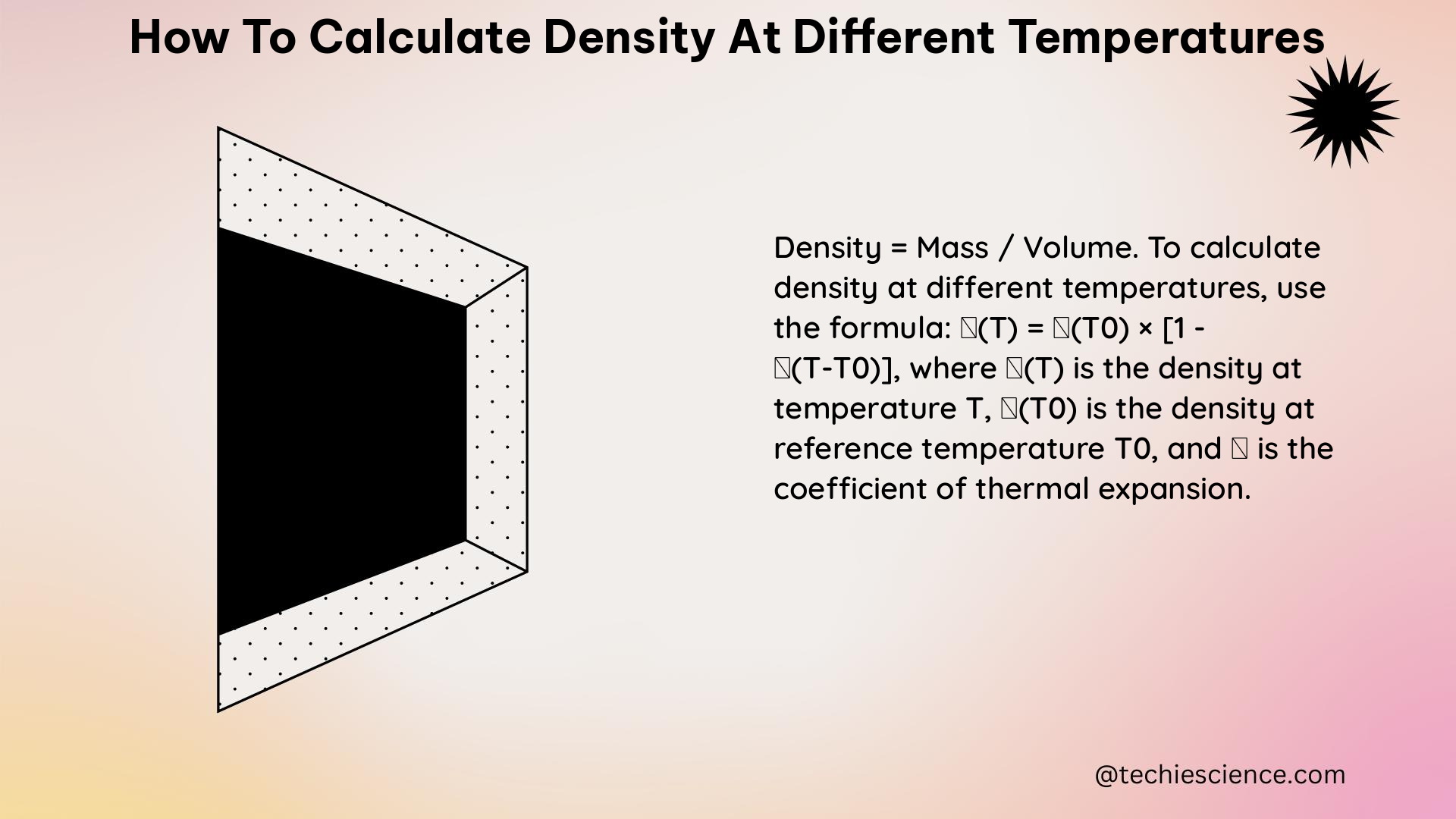
To calculate the density of a liquid at a different temperature, follow these steps:
-
Find the Temperature Difference: Subtract the final temperature from the initial temperature in degrees Celsius.
-
Apply the Volumetric Temperature Expansion Coefficient: Multiply the temperature difference by the volumetric temperature expansion coefficient for the specific substance. The volumetric temperature expansion coefficient represents the fractional change in volume per degree Celsius. For water, this coefficient is 0.0002 m³/m³°C, and for ethyl alcohol, it is 0.0011 m³/m³°C.
-
Adjust the Initial Density: Add 1 to the product of the temperature difference and the volumetric temperature expansion coefficient. Then, divide the initial density by this result to find the final density at the new temperature.
The formula for calculating the final density of a liquid at a different temperature can be expressed as:
ρ_final = ρ_initial / (1 + β × ΔT)
Where:
– ρ_final is the final density at the new temperature (kg/m³)
– ρ_initial is the initial density at the reference temperature (kg/m³)
– β is the volumetric temperature expansion coefficient (m³/m³°C)
– ΔT is the temperature difference (°C)
Example Calculation for Liquid Density
Let’s consider an example of calculating the density of water at different temperatures:
- Initial temperature: 30°C
- Final temperature: 20°C
- Initial density: 1000 kg/m³
-
Volumetric temperature expansion coefficient for water: 0.0002 m³/m³°C
-
Calculate the temperature difference:
ΔT = 30°C - 20°C = 10°C -
Apply the volumetric temperature expansion coefficient:
Adjustment factor = 1 + (10°C × 0.0002 m³/m³°C) = 1.002 -
Calculate the final density:
ρ_final = 1000 kg/m³ / 1.002 ≈ 998.01 kg/m³
Therefore, the density of water decreases from 1000 kg/m³ at 30°C to approximately 998.01 kg/m³ at 20°C.
Calculating Density of Gases at Different Temperatures
To calculate the density of a gas at a different temperature, follow these steps:
-
Convert Temperature to Kelvin: Add 273.15 to the temperature in degrees Celsius to convert it to Kelvin.
-
Use the Ideal Gas Law: The ideal gas law relates the pressure, volume, amount of substance, and absolute temperature of a gas. The formula for the density of a gas is:
ρ = (P × MM) / (R × T)
Where:
– ρ is the density of the gas (kg/m³)
– P is the pressure of the gas (Pa)
– MM is the molar mass of the gas (kg/mol)
– R is the gas constant (e.g., 287.05 J/(kg·K) for dry air)
– T is the absolute temperature of the gas (K)
Example Calculation for Gas Density
Let’s consider an example of calculating the density of dry air at a specific temperature and pressure:
- Temperature: 10°C = 283.15 K
- Pressure: 10,000 Pascals
- Gas constant for dry air: 287.05 J/(kg·K)
Applying the formula for the density of a gas:
ρ = (10,000 Pa × 0.02897 kg/mol) / (287.05 J/(kg·K) × 283.15 K)
ρ ≈ 0.819 kg/m³
Therefore, the density of dry air at 10°C and 10,000 Pascals is approximately 0.819 kg/m³.
Key Formulas and Coefficients
Here are the key formulas and coefficients you need to know for calculating density at different temperatures:
Density Formula
ρ = m / V
Where:
– ρ is the density (kg/m³)
– m is the mass (kg)
– V is the volume (m³)
Ideal Gas Law
PV = nRT
Where:
– P is the pressure (Pa)
– V is the volume (m³)
– n is the amount of substance (mol)
– R is the gas constant (J/(mol·K))
– T is the absolute temperature (K)
Density of Gas
ρ = (P × MM) / (R × T)
Where:
– ρ is the density of the gas (kg/m³)
– P is the pressure of the gas (Pa)
– MM is the molar mass of the gas (kg/mol)
– R is the gas constant (J/(kg·K))
– T is the absolute temperature of the gas (K)
Volumetric Temperature Expansion Coefficient
- Water: 0.0002 m³/m³°C
- Ethyl alcohol: 0.0011 m³/m³°C
Gas Constant
- Dry air: 287.05 J/(kg·K)
Conclusion
Calculating the density of substances at different temperatures is a fundamental skill in various scientific and engineering fields. By understanding the relationship between temperature and density, as well as the key formulas and coefficients, you can accurately determine the density of both liquids and gases under different temperature conditions.
Remember to always convert the temperature to the appropriate units (Celsius or Kelvin) and use the correct volumetric temperature expansion coefficient or gas constant for the specific substance. With the information provided in this comprehensive guide, you should now be well-equipped to tackle any density calculation problem involving temperature changes.
References
- Sciencing. (2020). How to Calculate Densities at Various Temperatures. Retrieved from https://sciencing.com/calculate-densities-various-temperatures-5999071.html
- Scribd. (n.d.). How to Calculate Densities at Various Temperatures. Retrieved from https://www.scribd.com/doc/216655913/How-to-Calculate-Densities-at-Various-Temperatures
- LibreTexts. (2023). Density and Percent Compositions. Retrieved from https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_%28Analytical_Chemistry%29/Quantifying_Nature/Density_and_Percent_Compositions
- Study.com. (n.d.). Density of Gas | Overview, Formula & Examples. Retrieved from https://study.com/academy/lesson/how-to-find-the-density-of-a-gas.html
- Physics Stack Exchange. (2014). Density as a function of Temperature? Retrieved from https://physics.stackexchange.com/questions/102806/density-as-a-function-of-temperature

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.