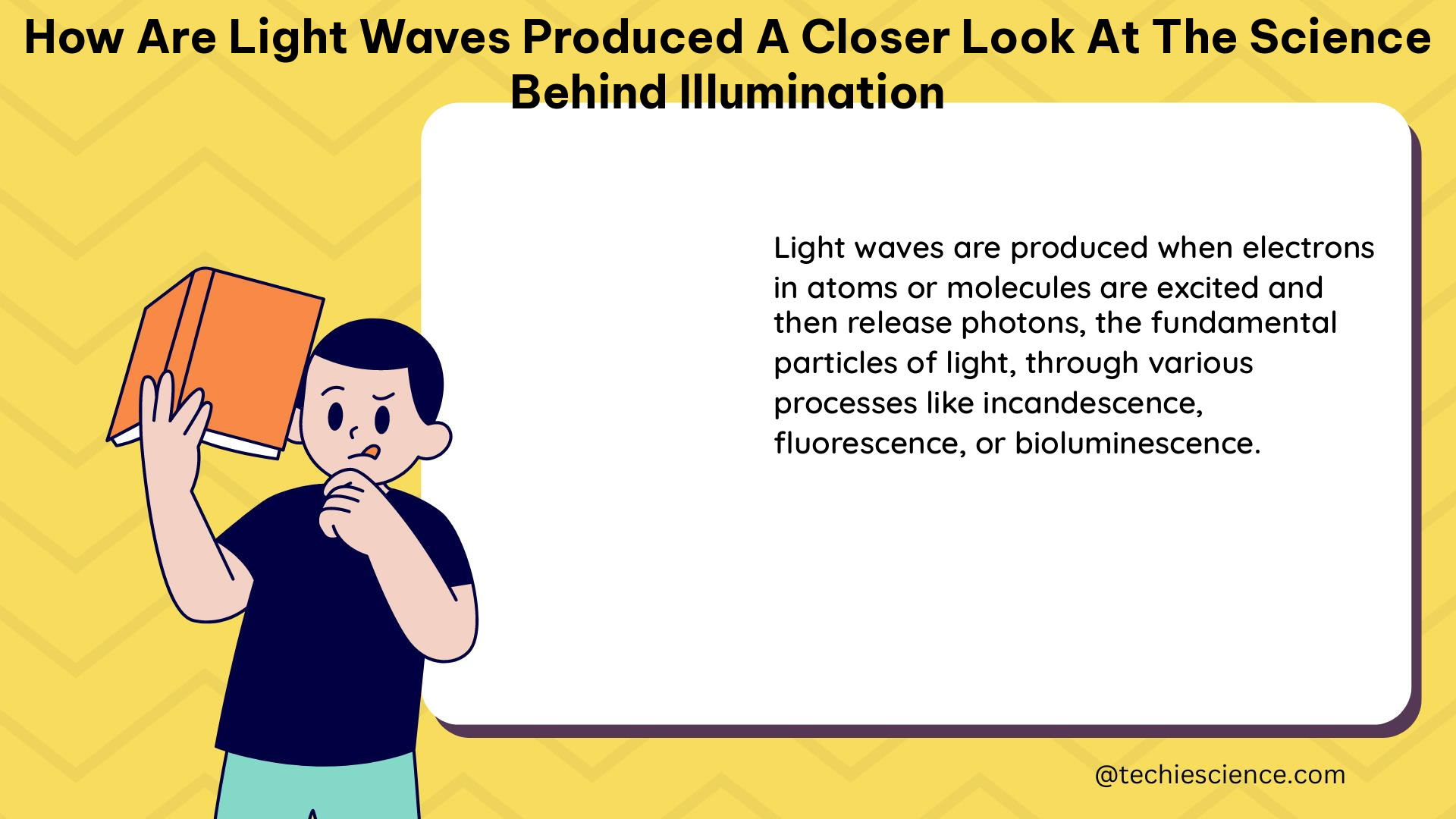
Summary
Light waves are produced through the oscillation of charged particles, such as electrons, which create oscillating electric and magnetic fields. This oscillation results in the emission of electromagnetic radiation in the form of light waves. The frequency and wavelength of these light waves determine their color and other properties. Understanding the science behind the production of light waves is crucial for various applications, from lighting and display technologies to medical imaging and telecommunications.
The Fundamentals of Light Wave Production
Charged Particle Oscillation
The production of light waves is primarily driven by the oscillation of charged particles, such as electrons, within atoms or molecules. When these charged particles are accelerated or decelerated, they create oscillating electric and magnetic fields, which in turn generate electromagnetic radiation in the form of light waves.
The frequency of the oscillation determines the wavelength and frequency of the resulting light waves, as described by the equation:
c = λν
where c is the speed of light, λ is the wavelength, and ν is the frequency.
Electromagnetic Radiation and the Electromagnetic Spectrum
The oscillation of charged particles produces electromagnetic radiation, which includes a wide range of wavelengths and frequencies, collectively known as the electromagnetic spectrum. This spectrum encompasses various forms of radiation, including radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays.
The visible light portion of the electromagnetic spectrum, which is the range of wavelengths that the human eye can detect, spans from approximately 400 nanometers (nm) to 700 nm, corresponding to a frequency range of 430 terahertz (THz) to 750 THz.
Factors Influencing Light Wave Production
The production of light waves can be influenced by various factors, including:
-
Temperature: At higher temperatures, the increased thermal energy of atoms and molecules can lead to more intense oscillation of charged particles, resulting in the emission of more energetic light waves, such as visible light or ultraviolet radiation.
-
Chemical Reactions: Chemical processes that involve the rearrangement of atoms and the release of energy can also lead to the production of light waves. For example, the burning of wood or the combustion of fuels can result in the emission of light in both the infrared and visible ranges.
-
Electric Fields: The application of strong electric fields can accelerate charged particles, causing them to oscillate and emit electromagnetic radiation, including light waves.
-
Atomic and Molecular Transitions: When atoms or molecules transition between different energy levels, they can emit or absorb specific wavelengths of light, which is the basis for many light-producing mechanisms, such as fluorescence and phosphorescence.
Quantifiable Data Points
Here are some key quantifiable data points related to the production of light waves:
- Wavelength of Visible Light: Approximately 400-700 nm
- Frequency of Visible Light: Approximately 430-790 THz
- Speed of Light: Approximately 299,792 km/s or 186,282 mi/s
- Amplitude of a Light Wave: Directly related to the intensity or brightness of the light
- Relationship between Frequency and Wavelength:
c = λν
The Physics Behind Light Wave Production
Electromagnetic Wave Theory
The production of light waves is explained by the electromagnetic wave theory, which states that oscillating electric and magnetic fields can propagate through space in the form of electromagnetic radiation, including light waves.
The fundamental equations that describe the behavior of electromagnetic waves are Maxwell’s equations, which were developed by the physicist James Clerk Maxwell in the 19th century. These equations describe the relationships between the electric and magnetic fields, as well as their interactions with charged particles and currents.
Quantum Mechanics and Light Wave Production
At the atomic and molecular level, the production of light waves is also governed by the principles of quantum mechanics. When electrons in atoms or molecules transition between different energy levels, they can emit or absorb specific wavelengths of light, corresponding to the energy difference between the levels.
This process is known as atomic or molecular transitions, and it is the basis for many light-producing mechanisms, such as:
- Fluorescence: Occurs when an atom or molecule absorbs a photon of light and then re-emits a photon of a different wavelength.
- Phosphorescence: A process where an atom or molecule absorbs a photon of light and then slowly re-emits the energy as a photon of a different wavelength.
- Incandescence: The emission of light due to the thermal energy of an object, such as a heated filament in a light bulb.
Numerical Examples and Calculations
To illustrate the relationship between the frequency, wavelength, and speed of light, let’s consider a few numerical examples:
- Visible Light Wavelength and Frequency:
- Wavelength range of visible light: 400 nm to 700 nm
- Frequency range of visible light: 430 THz to 750 THz
-
Using the equation
c = λν, we can calculate the frequency for a specific wavelength:- For a wavelength of 500 nm, the frequency would be
c / λ = 299,792 km/s / 500 nm = 599.58 THz
- For a wavelength of 500 nm, the frequency would be
-
Speed of Light Calculation:
- The speed of light in a vacuum is approximately 299,792 km/s or 186,282 mi/s.
-
This value can be derived from the relationship
c = λν, wherecis the speed of light,λis the wavelength, andνis the frequency. -
Amplitude and Intensity of Light Waves:
- The amplitude of a light wave is directly related to its intensity or brightness.
- A higher amplitude corresponds to a brighter light, while a lower amplitude corresponds to a dimmer light.
- The intensity of a light wave is proportional to the square of its amplitude, as described by the equation
I = A^2, whereIis the intensity andAis the amplitude.
These examples demonstrate how the fundamental equations and relationships in physics can be used to understand and quantify the properties of light waves.
Practical Applications of Light Wave Production
The understanding of how light waves are produced has led to numerous practical applications in various fields, including:
-
Lighting and Illumination: The production of light waves is the basis for various lighting technologies, such as incandescent bulbs, fluorescent lamps, and LED lights, which are used for indoor and outdoor illumination.
-
Display Technologies: The controlled production of light waves is essential for the development of display technologies, such as LCD, OLED, and plasma displays, which are used in televisions, computer monitors, and mobile devices.
-
Medical Imaging: Light waves, particularly in the infrared and visible ranges, are used in various medical imaging techniques, such as endoscopy, optical coherence tomography, and photodynamic therapy.
-
Telecommunications: The ability to transmit information using light waves has revolutionized the field of telecommunications, enabling high-speed data transmission through fiber-optic cables and free-space optical communication.
-
Scientific Research: The production and manipulation of light waves are crucial for various scientific research applications, such as spectroscopy, interferometry, and laser-based experiments.
Conclusion
The production of light waves is a fundamental aspect of our understanding of the physical world and has led to numerous technological advancements. By exploring the science behind the oscillation of charged particles and the resulting emission of electromagnetic radiation, we can gain a deeper appreciation for the complex and fascinating nature of light and its many applications.
References
- How is light as electromagnetic waves produced?
- The Production of Light
- Light and the Electromagnetic Spectrum
- Maxwell’s Equations
- Quantum Mechanics and Light

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.