Aluminium sulphide is a colourless chemical compound with interesting structure chemistry. Let us see how aluminium sulphide and nitric acid react with the help of article below.
Aluminium sulphate (Al2S3) and nitric acid react to form sulphur, water, nitric oxide and aluminium nitrate. Nitric acid (HNO3) is a strong acid that is a highly corrosive metal acid. Aluminium nitrate contains several crystal forms from which α, ß and Γ are stable. Nitric acid is also a commonly used oxidising agent.
We will focus on key facts about HNO3 + Al2S3 reaction, like redox reaction, type of reaction, balanced chemical equation and products in this article.
What is the product of HNO3 and Al2S3
Aluminium nitrate [Al(NO3)3], Nitric oxide (NO), water (H2O) and sulfur (S) are the compounds obtained for HNO3 and Al2S3 reaction. The chemical equation for the reaction is,
HNO3 + Al2S3 ͘= Al(NO3)3 + NO + H2O + S
What type of reaction is HNO3 + Al2S3
HNO3 + Al2S3 is an oxidation-reduction reaction.
How to balance HNO3 + Al2S3
The balanced chemical equation for HNO3 + Al2S3 is,
8HNO3 + Al2S3 = 2Al(NO3)3 + 2NO + 4H2O + 3S
- The general unbalanced chemical equation is,
- HNO3 + Al2S3 ͘= Al(NO3)3 + NO + H2O + S
- Noted the number of moles of atoms present on the reactant as well as the product side. The number of atoms is as given below,
| Atoms | Number on reactant side | Number on product side |
|---|---|---|
| H | 1 | 2 |
| N | 1 | 4 |
| O | 3 | 11 |
| Al | 2 | 1 |
| S | 3 | 1 |
- The equation is balanced by multiplying HNO3, Al(NO3)3, NO, H2O and S by the coefficient of 8, 2, 2, 4 and 3, respectively. The coefficients are obtained by comparing the table above and the hit-and-trial method.
- Thus, the balanced chemical equation is,
- 8HNO3 + Al2S3 = 2Al(NO3)3 + 2NO + 4H2O + 3S
HNO3 + Al2S3 titration
The titration between HNO3 and Al2S3 is not feasible as Al2S3 does not work as a strong electrolyte; thus, there is not much change in the pH of the solution, even on excess addition. And no indicator is available to work on such slow progress.
HNO3 + Al2S3 net ionic equation
The net ionic equation for HNO3 and Al2S3 reaction is,
8H+ (aq.) + 2NO3– (aq.) + Al2S3 (aq.) = 2Al+ (aq.) + 2NO (g) + 4H2O (l) + 3S (s)
- Write the general balanced chemical equation,
- 8HNO3 + Al2S3 = 2Al(NO3)3 + 2NO + 4H2O + 3S
- Indicate each compound with its chemical state (s, l, g or aq).
- 8HNO3 (aq.) + Al2S3 (aq.) = 2Al(NO3)3 (aq.) + 2NO (g) + 4H2O (l) + 3S (s)
- Split the strong electrolytes into their respective ions in an aqueous solution to get the complete ionic equation.
- 8H+ (aq.) + 8NO3– (aq.) + Al2S3 (aq.) = 2Al+ (aq.) + 6NO3– (aq.) + 2NO (g) + 4H2O (l) + 3S (s)
- Eliminate spectator ions to obtain the net ionic equation.
- 8H+ (aq.) + 2NO3– (aq.) + Al2S3 (aq.) = 2Al+ (aq.) + 2NO (g) + 4H2O (l) + 3S (s)
HNO3 + Al2S3 conjugate pairs
HNO3 and Al2S3 have the following conjugate pairs,
- The conjugate pair of HNO3 is its conjugate base NO3–.
- The conjugate base of H2O is OH–.
HNO3 and Al2S3 intermolecular forces
HNO3 and Al2S3 have the following intermolecular forces,
- The intermolecular forces between HNO3 molecules are dipole-dipole interaction and London dispersion forces.
- Ionic bonds are present in Al(NO3)3 as it is an ionic compound.
- NO2 molecules contain dipole-dipole forces.
- Hydrogen bonding and dipole-dipole forces are present in H2O molecules.
Is HNO3 + Al2S3 a buffer solution
HNO3 + Al2S3 is not a buffer solution as HNO3 is a strong acid, but to prepare a buffer solution, weak acid must be needed with its conjugate base.
Is HNO3 + Al2S3 a complete reaction
HNO3 + Al2S3 reaction is a complete reaction and no other steps are left.
Is HNO3 + Al2S3 an exothermic or endothermic reaction
HNO3 + Al2S3 reaction is exothermic reaction as a strong acid is reacting with weak base and acid-base reactions are always accompanied by evolution if heat.
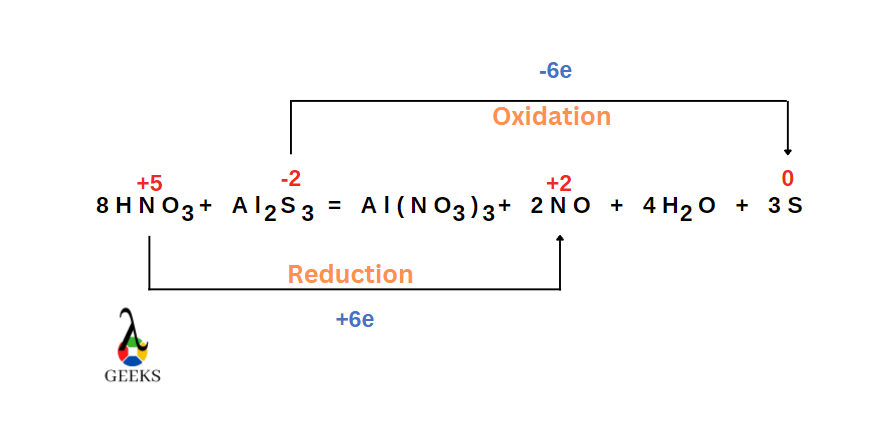
Is HNO3 + Al2S3 a redox reaction
HNO3 + Al2S3 reaction is a redox reaction in which sulfur is oxidised and nitrogen is reduced.
Is HNO3 + Al2S3 a precipitation reaction
HNO3 + Al2S3 reaction is a precipitation reaction and yellow sulphur precipitates are obtained.
Is HNO3 + Al2S3 reversible or irreversible reaction
HNO3 + Al2S3 reaction is an irreversible reaction because the sulphur precipitates do not dissolve under normal temperature-pressure conditions to give reactants back.
Is HNO3 + Al2S3 displacement reaction
HNO3 + Al2S3 reaction is not a displacement reaction but a redox reaction with several products formed by providing two reactants.
Conclusion:
The article concludes that HNO3 and Al2S3 are used to synthesize aluminium nitrate [Al(NO3)3]. Some ions do not completely ionize in their aqueous form, so they contribute to the net ionic equation. Conjugate pairs of only H2O and HNO3 are present.
Read more about following HNO3:

Hi, I am Sahil Singh. I completed my graduation in Bachelor of Science. I always have keen interest in Physics & Chemistry. I worked on my own blog for 1 year in the technology and gaming niche. I try my best to provide valuable knowledge through my articles.
You can reach out to me on LinkedIn: