In a chemical reaction chemical symbols are used to represent reactant and product. Let us discuss about the reaction of HI and Sr(OH)2.
Hydrogen iodide or HI is a gas but when dissolved in water forms the strongest hydro iodic acid. Strontium hydroxide or Sr(OH)2 is colorless solid substance which is Deliquescent in nature. The dipole moment of HI is 0.38 D with 169 pm bond length. Sr(OH)2 can absorbs CO2 very easily.
Strontium hydroxide is insoluble in acetone with molar mass 121.63 g/mol. Let us discuss about HI+ Sr(OH)2 with all details.
What is the product of HI and Sr(OH)2
HI reacts with Sr(OH)2to form strontium iodide , SrI2 and water.
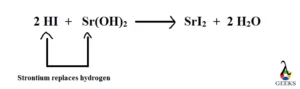
2HI + Sr(OH)2 ——> SrI2 + 2H2O
What type of reaction is HI + Sr(OH)2
HI + Sr(OH)2 is a double displacement reaction. In this reaction hydrogen is displaced from hydrogen iodide by strontium to yield strontium iodide and water.
How to balance HI + Sr(OH)2
After balancing a chemical reaction there will be equal number of atoms on both the reactant and product side. Following is the reaction balancing steps.
- Assigning certain coefficients to both reactants and products
- a HI + b Sr(OH)2 ——-> c SrI2 + d H2O
- An equation is made with the above equation
- H= a= 2b= 2d, I=a =2c, Sr =b = c, O=2b =d
- The linear equation is a +2b= 2d,a=2c,b=c,2b =d
- Solve the above equation using gauss elimination method
- The values of coefficients is a =2, b= 1, c= 1, d= 2
- The balanced equation of HI + Sr(OH)2 is
- 2HI + Sr(OH)2 ——-> SrI2 + 2H2O
HI + Sr(OH)2 titration
HI + Sr(OH)2 titration can be performed. It is because one is strong acid and other one is a strong base. So they can be titrated against to get the endpoint sharply.
Apparatus
Conical flask, pipette, burette, HI and Sr(OH)2 solution.
Indicator
Methyl orange and phenolphthalein is used because we are titrating strong acid and strong base.
Procedure
- Desired amount of strontium hydroxide is pipetted into a conical flask and burette is filled with HI.
- Methyl orange indicator or phenolphthalein is added into the solution in conical flask and titrate against HI in burette.
- If methyl orange is using the color change is from yellow to red and in case of phenolphthalein it is pink to colorless.
- Note down the burette reading and do the respective calculations.
HI + Sr(OH)2 net ionic equation
The net ionic equation of HI and Sr(OH)2
H+ + OH– ——-> H2O
- The ionic dissociation of HI, Sr(OH)2 and SrI2 is
- H+ + I– ——-> HI
- Sr2+ + 2OH– ——–> Sr(OH)2
- Sr2+ + 2I– ———> SrI2 .
HI + Sr(OH)2 conjugate Pairs
HI + Sr(OH)2 has conjugate pairs,
- The Conjugate base of HI is I– or iodide.
- The Conjugate acid of HI is H2I+.
- There is no Conjugate acid observed for Sr(OH)2.
HI and Sr(OH)2 intermolecular forces
The intermolecular forces exist in between HI + Sr(OH)2 is,
- In HI there is ionic force of attraction and dipole-dipole interaction can be seen in between H+ ion and I– .
- In Sr(OH)2 electrostatic force of attraction observed between the ions.
HI + Sr(OH)2 reaction enthalpy
The reaction enthalpy of HI + Sr(OH)2 is 83.6 KJ/mol. The enthalpy of the formation of HI, SrI2 , H2O and Sr(OH)2 is 26.5, -561.4,-285.8 and -957.3 kJ/mol respectively. Hence enthalpy is {-561.4 + -285.8-(26.5+-957.3)}.The reaction is found to be endothermic because the reaction enthalpy value is positive
Is HI + Sr(OH)2 a buffer solution
HI + Sr(OH)2 is not a buffer solution. In a buffer solution, there is a mixture of weak acid with conjugate base or vice versa. Here both the reactants are strong acid and base. So there mixture will not comprise a buffer solution.
Is HI + Sr(OH)2 a complete reaction
HI + Sr(OH)2 is a complete reaction. The reaction takes place at room temperature to yield strontium iodide and water. Sr2+ ion displaces Hydrogen to form the products.
Is HI + Sr(OH)2 an exothermic or endothermic reaction
HI + Sr(OH)2 is an endothermic reaction. The reaction doesn’t liberate any amount of heat. So it is an endothermic reaction.
Is HI + Sr(OH)2 a redox reaction
HI + Sr(OH)2 is not a redox reaction. In a redox reaction both oxidation and reduction can occur. So the oxidation state of atoms in reactant side and product side will be different. But here the oxidation state of all the atoms are same before and after the reaction. So it’s is not redox reaction.
Is HI + Sr(OH)2 a precipitation reaction
HI + Sr(OH)2 is reaction doesn’t form any precipitate. It is because the product formed strontium iodide is soluble in water will get dissolved and not precipitated.
Is HI + Sr(OH)2 reversible or irreversible reaction
HI + Sr(OH)2 is an irreversible reaction. This reaction can’t be reversed to yield it’s reactants
Is HI + Sr(OH)2 a displacement reaction
HI + Sr(OH)2 is a double displacement reaction because the cations and anions of both the reactants are interchanged to yield the products.
Conclusion
Hydrogen iodide is used for many organic reactions to convert primary alkyl halides. It is a good source of iodine. Strontium hydroxide is used as stabilizer and a good source of strontium.

Hi… I am Aparna Dev, a chemistry Postgraduate with a good understanding of chemistry concepts. I am working in Kerala Minerals and Metals Limited Kollam with experience in the development of electrocatalysts as a part of post graduate thesis.
Let’s connect through LinkedIn-https://www.linkedin.com/in/aparna-dev-76a8751b9