HF is a weak acid, and ZnCO3 is a weak base. Let us dive into some facts about this HF + ZnCO3 reaction.
HF is an inorganic compound also known as fluorane. It is a colorless gas or liquid, and its melting point is -83. 6°C.It is used in the petrochemical industry and is corrosive when mixed with moisture. ZnCO3 is a white crystalline solid which is insoluble in water. It occurs from smithsonite metal.
This article will discuss some of the characteristics of this HF + ZnCO3 reaction, like its products formed during the reaction molecular forces present in the.
What is the product of HF and ZnCO3
Zinc fluoride, Carbon dioxide and water are formed during the reaction of HF and ZnCO3.
ZnCO3 + 2HF —> ZnF2 + CO2 + H2O
What type of reaction is HF + ZnCO3
HF + ZnCO3 is an acid-base reaction.
How to balance HF + ZnCO3
The reaction HF + ZnCO3 gets balanced using the following steps.
ZnCO3 + 2HF —> ZnF2 + CO2 + H2O
- Count the number of atoms on both the reactants and products side.
- The below table gives us the information about the number of atoms present on both reactants and product sides.
| Atoms | Reactants Side | Products Side |
|---|---|---|
| Zn | 1 | 1 |
| C | 1 | 1 |
| O | 3 | 3 |
| H | 1 | 2 |
| F | 1 | 2 |
- Placing the stoichiometric numbers in front of unbalanced atoms. The reaction gets balanced by placing 2 as a coefficient of HF.
- Therefore, the balanced chemical equation for t HF + ZnCO3 is as follows;
- ZnCO3 + 2HF —> ZnF2 + CO2 + H2O
HF + ZnCO3 titration
We can perform a titration of HF + ZnCO3. We will see how this titration workup.
Apparatus
Conical flasks, Volumetric flasks, Burette, Pipette, measuring jars, Burette Stand, Wash Bottle, Spatula, Weighing bottle.
Indicator
Phenolphthalein indicator is used in acid-base titration; its endpoint is pale pink to colorless.
Procedure
The burette is filled with HF, and the conical flask is filled with ZnCO3.Starts the titration by the dropwise addition of HF and add indicator. The point at which color disappears is the equivalent point. Note the reading and find the volume of ZnF2 by using the formula V1S1=V2S2.
HF + ZnCO3 net ionic equation
The net ionic equation for the HF + ZnCO3 reaction is
ZnCO3(aq) +2H+(aq) –> Zn2+(aq) + CO2(g) + H2O(I)
- Write the balanced chemical equation.
- The balanced chemical equation for the HF + ZnCO3 reaction is as follows;
- ZnCO3(aq) + 2HF (aq) —> ZnF2(aq) + CO2 (g) + H2O(I)
- Splitting the strong electrolytes into ions. Here ZnCO3 is not a strong electrolyte.
- ZnCO3 +2H+ +2F– —> Zn2+ + 2F– + CO2 + H2O
- Cancel the spectator ions on both sides gives the net ionic equation.
- ZnCO3 +2H+ –> Zn2+ + CO2 + H2O
- Therefore, the net ionic equation for the HF + ZnCO3 reaction is as follows;
- ZnCO3 +2H+ –> Zn2+ + CO2 + H2O
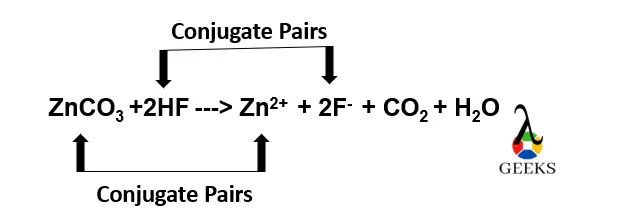
HF + ZnCO3 conjugate pairs
HF + ZnCO3 reaction has the following conjugate pairs,
- · HF and F – are the conjugate acid-base pairs formed in HF + ZnCO3 reaction.
- ZnCO3 and Zn2+ are the conjugate acid-base pairs formed in HF + ZnCO3 reaction.
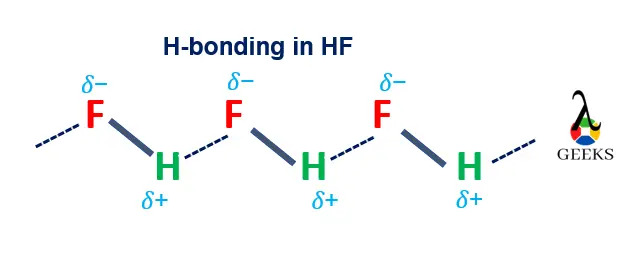
HF and ZnCO3 intermolecular forces
HF + ZnCO3 reaction has the following intermolecular forces,
- Dipole-Dipole interactions, Hydrogen bonding, London Dispersion intermolecular forces present in HF.
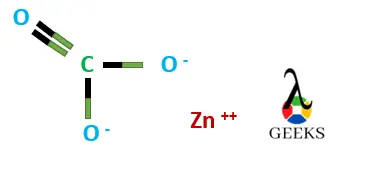
- Ion -Ion intermolecular forces present in ZnCO3.
HF + ZnCO3 reaction enthalpy
Enthalpy of HF + ZnCO3 is not available in the literature because of stochiometry.
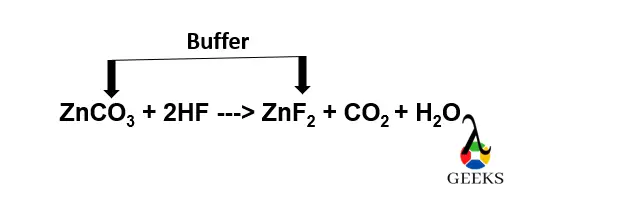
Is HF + ZnCO3 a buffer solution
HF + ZnCO3 is a buffer solution since HF is a weak acid, its conjugate base is F–, ZnCO3 is a weak base, and its conjugate acid is Zn2+.
Is HF + ZnCO3 a complete reaction
HF + ZnCO3 is a complete reaction because the products formed during this reaction having no tendency to react further.
Is HF + ZnCO3an exothermic or endothermic reaction
The literature does not find the exothermic or endothermic behavior of HF + ZnCO3.
Is HF + ZnCO3 a redox reaction
HF + ZnCO3 is not a redox reaction because no transfer of electrons was observed during this reaction.
Is HF + ZnCO3 a precipitation reaction
HF + ZnCO3 is not a precipitation reaction since no precipitate formation is observed during the reaction.
Is HF + ZnCO3 reversible or irreversible reaction
HF + ZnCO3 is not a reversible reaction as the products formed during the reaction no longer undergo any backward reaction under the same condition.
Is HF + ZnCO3 displacement reaction
HF + ZnCO3 is a displacement reaction as Zn displaces from ZnCO3 to ZnF2.
Conclusion
HF+ ZnCO3 reaction is the source for ZnF2, which is used for fluorinating many organic compounds. It is used to make fluorescent lights and preserve wood, mainly in ceramic manufacturing. Electroplating baths are also prepared by using this ZnF2. It plays an important role in the galvanizing of steel.
Read more about following HF facts

Hi…I am Surya Satya Eluri. I have done my M.Sc in Organic Chemistry. I am very enthusiastic about the high-energy chemistry field. I love to write complicated chemistry concepts in understandable and simple words.
Let’s connect through LinkedIn: