Hydrofluoric acid reacts with bromine to give hydrobromic acid and fluorine gas. Let us see the detailed reactions between HF and Br2 shown below.
Hydrofluoric acid, the aqueous solution of HF, is a colorless and highly corrosive compound widely used to prepare chlorinated compounds and used in Teflon. Bromine (Br2) is an element with the atomic number 35 . Br2 belongs to group-17 of the period table.
Let us see the product formed, the type of reaction, the reversible or Irreversible reaction, net ionic equation of HF + Br2 in detail.
What is the product of HF and Br2?
hydrogen bromide (HBr) and fluorine (F2) are formed when Hydro fluoride (HF) reacts with bromine (Br2).
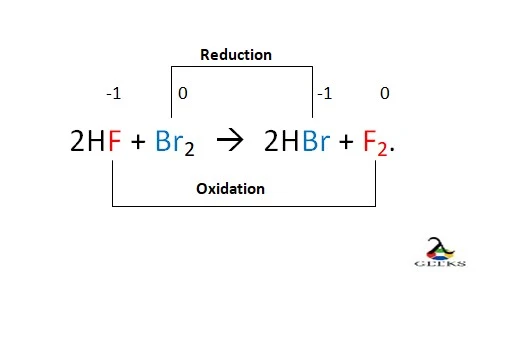
HF + Br2 –> HBr + F2.
What type of reaction is HF + Br2?
HF + Br2 is a redox reaction in which F– oxidizes to F, and Br gets reduced to Br–.
2F– – 2e –> F [oxidation]
2Br –> 2Br– – 2e [reduction]
How to balance HF + Br2?
- The general equation is HF + Br2 –> HBr + F2.
- The reaction between HF and Br2 is balanced, as below:
- Now add an unknown Coefficient to each compound as shown below as: a HF + b Br2 –> c HBr + d F2
- Here, H = a = c, F = a = 2d , Br = 2b = c
- The number of moles of F and Br is not balanced. Therefore, to balance it put a is = 2 and C is = 2
- number of moles of each element is balanced on both sides.
- The balanced equation is
- 2 HF + Br2 –> 2HBr + F2.
HF + Br2 titration
The titration between HF and Br2 is carried out as shown below.
Apparatus used
Burette, conical flask, wash bottle, burette stand, volumetric flask, beakers.
Indicator
Methyl blue is the indicator used for the titration of bromine because a color change is noticed from pale blue to colorless.
Procedure
- The burette is filled with standardized Br2.
- HF is taken in a conical flask; add 2-3 drops of indicator, and the measure is noted.
- The Br2 in the burette is made to add to the conical flask dropwise with constant stirring.
- After some time, a color change is observed, denoting the endpoint.
- Repeat the above steps for three more values and note the final result.
- Calculate the quantity of bromine by using the formula V1S1= V2S2.
HF + Br2 net ionic equation
The net ionic equation of HF + Br2 is 2F–(aq) + Br2(l) –> 2Br– (aq) + F2(g).
- The state of elements and ionic equation of the HF + Br2 reaction is as follows:
- 2H+(aq) + 2F–(aq) + Br2(l) –> 2H+ (aq) + 2 Br–(aq) + F2(g).
- The same ions from either side get canceled. Therefore,
- 2F–(aq) + Br2(l) –> 2Br– (aq) + F2(g).
Is HF + Br2 a conjugate pair
HF + Br2 is conjugate pair because they differ in a Proton number to form a conjugate pair.
- The conjugate base of HF is F–
- The conjugate acid of Br– is HBr.
HI + Br2 intermolecular forces
- HF exhibits hydrogen bonds and dipole-dipole intermolecular forces.
- The intermolecular force in the Br2 molecule is the London-dispersion force and Vander Waals force.
- Dipole-dipole interaction is observed in HBr.
HF + Br2 reaction enthalpy
- The reaction enthalpy of HF + Br2 is 561.3 KJ/mol. The enthalpy of the formation of HF, Br2, HBr and F2 molecules is shown below:
| Molecules | Reaction enthalpy(KJ/mol) |
|---|---|
| HF | -332.3 |
| Br2 | 30.9 |
| HBr | -36.23 |
| F2 | 0 |
- The reaction enthalpy is calculated as follows.
- Reaction enthalpy = (standard enthalpy of products) – (standard enthalpy of reactants)
- Here, there is two moles each of HBr and HF is present.
- (-72.46)-(-633.81) = 561.3 KJ/mol
Is HF + Br2 a buffer solution
HF + Br2 gives a buffer solution as HF is a weak acid with its conjugate base. Adding strong acids or bases does not affect the pH of the solution.
Is HF + Br2 a complete reaction
The reaction between HF and Br2 is complete because no more reaction occurs after the products are formed.
Is HF + Br2 an exothermic or endothermic reaction
The reaction between HF and Br2 is endothermic reaction as the reaction enthalpy value is positive.
Is HF + Br2 a redox reaction
The reaction between HF and Br2 is a redox reaction because both oxidation and reduction take place.
Is HF + Br2 a reversible reaction
The reaction between HF and Br2 is irreversible as F2 formed evolves, and the product cannot be changed to reactants by any method.
Is HF + Br2 a precipitation reaction
The reaction between HF and Br2 is not a precipitation reaction because no precipitate is formed as a product.
Is HF + Br2 a displacement reaction
The reaction between HF and Br2 is a single displacement reaction in which the F atom gets displaced from HF to fluorine gas, and Br gets displaced to H from Br2.
Conclusion
The reaction between is a redox reaction in which HBr and fluorine gas are formed. This reaction helps produce fluorine gas, and it is used to produce other fluorinated compounds.

Hello…I am Manjula Sivapuri. I have completed my graduation in Chemistry. Currently working as a Chemistry Subject Matter expert in LambdaGeeks. My keen interest in chemistry has brought me to this platform to share my knowledge on the subject. I hope my work will make you understand the topics well.
Connect me via LinkedIn