The strong hydrochloric acid reacts with lead sulfite to produce white odorless solid. Let us study in detail the reaction of HCl+ PbSO3.
Hydrochloric acid (HCl) is a colorless transparent liquid with an irritating odor. The lead (II) sulfite, also known as scotlandite, is the first naturally occurring, greyish-white, transparent, colorless mineral sulfite. Further reaction of sulfurous anhydride and water leads to the formation of sulfurous acid.
The following article researches balancing chemical reactions, type of reaction, net ionic reaction, etc.
What is the product of HCl and PbSO3?
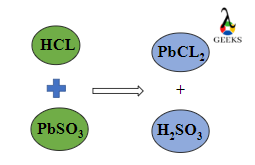
Hydrochloric acid (HCl) reacts with lead (II) sulfite (PbSO3) to form lead dichloride, sulfur dioxide, and water. Further SO2 reacts with H2O to form H2SO3.
PbSO3 + 2HCl → PbCl2 + SO2 + H2O→ PbCl2 + H2SO3
What type of reaction is HCl + PbSO3?
HCl +PbSO3 reaction is classified as a neutralization reaction since HCl+PbSO3 forms lead dichloride (PbCl2), which is a slightly soluble salt.
How to balance HCl + PbSO3?
Follow the below steps to balance a chemical reaction –
- Write the unbalanced chemical reaction: PbSO3 + HCl → PbCl2 + H2SO3
- Tabulate the number of moles as shown below:
| Compounds | Reactant Side | Product Side |
|---|---|---|
| Pb | 1 | 1 |
| S | 1 | 1 |
| Cl | 1 | 2 |
| O | 3 | 3 |
| H | 1 | 2 |
- It is said to be a balanced chemical reaction when the number of moles on the left side of the arrow equals the number of moles on the right side. Here, moles of hydrogen and chlorine atoms are unbalanced.
- We need to multiply 2 with HCl on the reactant side to balance the reaction.
- The Balanced chemical reaction is – PbSO3 + 2HCl → PbCl2 + H2SO3
HCl + PbSO3 titration
HCl + PbSO3 titration is not performed due to the absence of a base compound.
HCl + PbSO3 net ionic equation
The net ionic equation is: PbSO3 (s) + 2Cl–(aq) = SO32-(aq) + PbCl2(s).
The steps followed are:
- Determine the phase/state of each element.
- PbSO3(s) + 2HCl(aq) = H2SO3(aq) + PbCl2(s)
- The Complete ionic equation is written by separating ionic compounds into their respective ions
- PbSO3 (s) + 2H+(aq) + 2Cl–(aq) = 2H+(aq) + SO32-(aq) + PbCl2(s)
- The common ions present on either side of the arrow are removed.
- PbSO3 (s) +
2H+(aq)+ 2Cl–(aq) =2H+(aq)+ SO32-(aq) + PbCl2(s) - The net ionic equation includes the species involved in the reaction.
- The net ionic equation is written as–
- PbSO3 (s) + 2Cl–(aq) = SO32-(aq) + PbCl2(s)
HCl + PbSO3 conjugate pairs
The conjugate acid-base pairs for HCl +PbSO3 are –
- The conjugate base of HCl= Cl–
- Conjugate base of PbSO3 = SO3–
HCl and PbSO3 intermolecular forces
The intermolecular forces on HCl and PbSO3 are –
- London dispersion forces, dipole-dipole interaction, and hydrogen bonding acts on HCl because it is a polar covalent compound.
- Hydrogen bond and Dipole-dipole interaction act on PbSO3.
HCl + PbSO3 reaction enthalpy
The reaction enthalpy, for HCl + PbSO3 cannot be calculated, since enthalpy of PbSO3 is not determined.
Is HCl + PbSO3 a buffer solution?
The HCl +PbSO3 is not a buffer solution because it is reacted with strong HCl acid.
Is HCl + PbSO3 a complete reaction?
HCl +PbSO3 is a complete reaction because, at equilibrium, complete moles of reactant is converted into a product.
Is HCl + PbSO3 an exothermic or endothermic reaction?
The exothermic or endothermic reaction for HCl +PbSO3 can not be performed because it depends on the reaction enthaly, which is not determined.
Is HCl + PbSO3 a redox reaction?
HCl + PbSO3 is not a redox reaction since no change in the oxidation state of elements is observed during the reaction.
Is HCl + PbSO3 a precipitation reaction?
HCl + PbSO3 is a precipitation reaction since it leads to the formation of PbCl2, which is a slightly soluble salt.
Is HCl + PbSO3 reversible or irreversible reaction?
HCl + PbSO3 is an irreversible reaction and can be altered only when there is a drastic change in temperature or pressure.
Is HCl + PbSO3 displacement reaction?
HCl + PbSO3 shows a double displacement (metathesis) reaction. In this reaction, H2 is traded with SO3 to form H2SO3, and Cl is traded with Pb to form PbCL2.
Conclusion
Hydrochloric acid reacts with lead (II) sulfite to form plumbous chloride (PbCL2) and sulfurous acid. PbCl2 is primarily used in producing infrared transmitting glass, aurene glass, lead salts, etc. The sulfurous acid produced can be used to manufacture fertilizers, dyes, metallurgical processes, and petroleum refining.
Hi….I am Pratham Manish Shah, Pursuing an Integrated MTech degree in Chemical Engineering from the Institute of Chemical Technology Mumbai Marathwada Jalna. With Lamdageeks, I am Interested in learning ongoing education opportunities to maintain knowledge of emerging technologies and methods.
Let’s connect via LinkedIn: www.linkedin.com/in/pratham-shah-07