Hydrochloric acid is a solution of gases (H2 and Cl2) in water. Ammonium Bromide is a salt of HBr and has a saline taste. Let us now see how HCl + NH4Br reacts with each other.
When hydrogen bromide directly acts on ammonia, NH4Br formation takes place. HCl or hydrogen chloride is a colorless gas that has a very strong odor. NH4Br is a very weak acid in comparison with HCl (i,e strong acid).
Now in this article, we will discuss the HCl + NH4Br reaction, net ionic equations, reaction type, conjugate pairs, enthalpy, etc in detail.
What is the product of HCl and NH4Br
Hydrobromic acid (HBr) and Salammoniac (NH4Cl) are the products formed by the reaction of Ammonium Bromide(NH4Br) and Hydrogen Chloride(HCl).
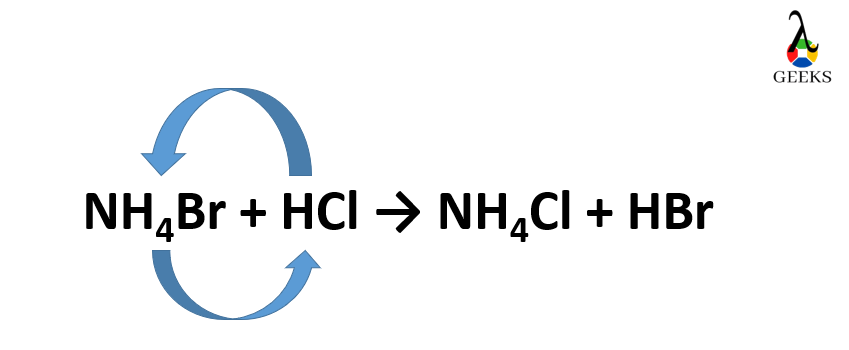
NH4Br + HCl → NH4Cl + HBr
What type of reaction is HCl +NH4Br
The HCl + NH4Br reaction is a double-displacement reaction or metathesis type of reaction.
NH4Br + HCl → NH4Cl + HBr
How to balance HCl+ NH4Br
The unbalanced equation for the reaction of HCl + NH4Br is written as-
NH4Br + HCl → NH4Cl + HBr
To balance the above equation we use the following steps-
- First, assign different variables for each of the compounds on both sides(i.e products and reactants).
- a NH4Br + b HCl → c NH4Cl + d HBr
- Several different equations are formed for each element, which represents the total number of atoms present.
- N; 1a =1c, H; 4a+1b= 4c +1d, Br; 1a=1d, Cl;1b=1c
- Now, by using the substitution or elimination method to solve the above equation for each variable, then we get-
- a=1(NH4Br), b=1HCl, c=1NH4Cl, d=1HBr
- Now count the number on both sides, we get-
| ELEMENTS | LHS | RHS |
| N | 1 | 1 |
| H | 5 | 5 |
| Br | 1 | 1 |
| Cl | 1 | 1 |
- Thus the balanced equation is,
- NH4Br + HCl → NH4Cl + HBr
HCl + NH4Br titration
Titration of HCl and NH4Br is not possible. As both the medium are acidic because the change in pH is not abrupt enough at either equivalence point to permit estimation with visual indicators.
HCl + NH4Br net ionic equation
The net ionic equation for the reaction of HCl + NH4Br is-
NH4Br (s) + H+(aq) + Cl–(aq) = NH4Cl(s) + H+(aq) + Br– (aq)
To get a net ionic equation following steps are used-
- First, write the complete inning equation as follows-
- NH4+(s) + Br–(s) + H+(aq) + Cl–(aq) = NH4+(s) + Cl–(s) + H+(aq) + Br– (aq)
- In this reaction H+ ion is a spectator ion, so it gets canceled from both sides.
- After, removing the H+ ion from both the product and reactant side the remaining equation is the net ionic equation of the reaction.
- Thus, the net ionic equation of the HCl + NH4Br reaction is –
- NH4Br (s) + Cl–(aq) = NH4Cl(s) + Br– (aq)
HCl + NH4Br conjugate pairs
In the reaction HCL +NH4Br the conjugate acid-base pairs are as follows-
- Conjugate base of HCl = Cl –
- Conjuagte acid of HCl = H3O+
- Conjugate base of NH4Br = NH4+
- Conjugate acid of NH4Br= Br-
HCl and NH4Br intermolecular forces
The reaction between HCl and NH4Br has the following intermolecular forces-
- Dipole-dipole interactions, and London dispersion forces are the two types of intermolecular forces present in HCl.
- In NH4Br, the ionic forces of attraction are present.
- The molecule of HBr has dipole-dipole forces as it is a polar molecule. There are also dispersion forces present. Among both of the forces dipole-dipole interactions, forces are stronger.
HCl + NH4Br reaction enthalpy
The reaction enthalpy of HCl + NH4Br is -28.1 kJ/mol. It is calculated as follows-
| Compound | Enthalpy of formation KJ/mol |
| NH4Br | -270.8 |
| HCl | -92.2 |
| NH4Cl | -314.4 |
| HBr | -20.2 |
- The total reaction enthalpy = ∑ ∆H0f (products) – ∑ ∆H0f (reactant)
- ∆H = (-314.4) + (-20.2) – (-270.8) + (-92.2) kJ/mol
- ∆H = -28.1 kJ/mol
Is HCl +NH4Br a buffer solution
When HCl and NH4Br are mixed, it does not form a buffer solution . As for the buffer solution, a weak acid and its weak salt are mixed but here, hydrochloride acid is present which is a very strong acid.
Is HCl + NH4Br a complete reaction
The reaction HCl +NH4Br is a complete reaction as HBr and Salammoniac are the products formed during the reaction and no further products are formed or the reaction cannot be carried on further.
Is HCl + NH4Br an exothermic or endothermic reaction
HCl + NH4Br is an exothermic reaction as the total enthalpy of formation is negative (-28.1 kJ/mol).
Is HCl + NH4Br a redox reaction
The reaction HCl + NH4Br is not a type of redox reaction, as there is no change in the oxidation number of any element on the both (product and reactant) sides takes place.
Is HCl + NH4Br a precipitation reaction
HCl + NH4Br is not a precipitation reaction as the products (ammonium chloride and hydrogen bromide) formed during this reaction are soluble and no precipitate is formed.
Is HCl + NH4Br reversible or irreversible reaction
HCl+ NH4Br is a reversible reaction as all the products formed in the reaction are soluble and no gas is evolved during the reaction which makes it convertible.
Is HCl + NH4Br displacement reaction
The HCl + NH4Br reaction is a displacement reaction as in this reaction, Br of NH4Br is displaced by Cl; and Cl from HCl is displaced by Br to form the respective products.
NH4Br + HCl → NH4Cl + HBr
Conclusion
Ammonium bromide is a white color powder, with 97.94 g/mol of molar mass. When it reacts with HCl gives ammonium chloride which is used in fertilizers and food additives etc. Hydrobromic acid (HBr) is generally used as a source in bromine manufacturing industries.

Hello…I am Prexa shah. I have recently completed my post-graduation in Chemistry and have almost 2 years of experience in academic writing.
You can connect with me on LinkedIn