HCl is a strong acid, whereas Mn2O7 is also an acid because of its highest oxidation state. Let us see some more facts about the reaction of HCl + Mn2O7.
In the reaction of HCl + Mn2O7, HCl is likely to completely dissociate into the aqueous media to form ions because of its acidic nature, producing an odd smell when it mixes with water. Mn2O7, known as manganese heptoxide, is a non-polar molecule. Its melting point is 5.9 °C, which can explode when we apply more heat.
In this article, we will find some of the characteristics of this reaction, like its products, conjugate pairs, and displacement reaction.
What is the product of HCl and Mn2O7
HCl reacts with Mn2O7 to produce manganous chloride (MnCl2), chlorine (Cl2), and water (H2O) as the by-product.
Mn2O7 + 14 HCl = 2 MnCl2 + 5Cl2 +7H2O
What type of reaction is HCl + Mn2O7
HCl + Mn2O7 is a type of acid-base reaction forming salt and water.
How to balance HCl + MN2O7
HCl + Mn2O7 reaction gets balanced as follows:
Step 1: Finding the number of atoms
- The table indicates the atoms and the number of atoms present in both reactants and products side of the reaction-
- Mn2O7 + HCl = MnCl2 + Cl2 +H2O
| Atoms | Reactants Side | Products Side |
|---|---|---|
| Mn | 2 | 1 |
| O | 7 | 1 |
| Cl | 2 | 4 |
| H | 1 | 2 |
Step 2: Place the coefficient in front of the atoms
- The reaction gets balanced by multiplying 4 with HCl and 2 with H2O and 2 with MnCl2.
- Mn2O7 + 4HCl = 2MnCl2 + Cl2 +2H2O
Step 3: Placing the required number as a coefficient before the molecule
- Multiplying HCl , MnCl2 , H20, and Cl2 by 14,2,7 and 5 makes the reaction balanced.
- Mn2O7 + 14 HCl = 2 MnCl2 + 5Cl2 +7H2O
HCl + Mn2O7 titration
HCl and Mn2O7 cannot be titrated together because HCl reacts with Mn2O7 to produce Cl2. This Cl2 will interfere during the titration.
HCl + Mn2O7 net ionic equation
- Split the strong electrolytes into ions on both reactant’s side and the products side, then after combining the ions to get the net ionic equation.
- The net ionic equation for the reaction of HCl+Mn2O7 is given by;
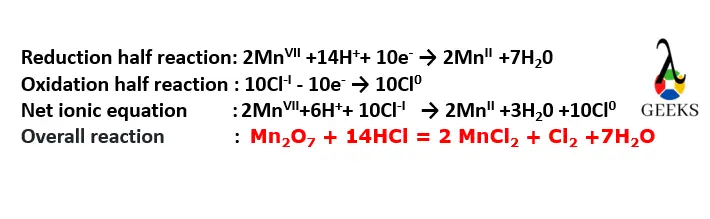
HCl + Mn2O7 conjugate pairs
- The conjugate base of HCl is Cl–.
- Mn2O7 does not have any conjugate pairs because of its oxidizing property.

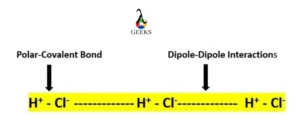
HCl and Mn2O7 intermolecular forces
- Dipole-Dipole interactions and London Dispersion forces are present in the HCl molecule, out of which dipole-dipole interactions are the strongest.
- Vander Waal forces are present in between Mn2O7 because of its covalent bonding.
HCl + Mn2O7 reaction enthalpy
The prediction of enthalpy for the reaction of HCl + Mn2O7 is impossible because of the strong oxidizing nature of Mn2O7.
Is HCl + Mn2O7 a buffer solution
HCl + Mn2O7 is not a buffer solution because HCl and Mn2O7 are not weak acids and so neither reactants form any buffer.
Is HCl + Mn2O7 a complete reaction
HCl + Mn2O7 is a complete reaction because the products formed during the reaction does not undergo any further reaction.
Is HCl + Mn2O7 an exothermic or endothermic reaction
We cannot predict the exothermic or endothermic nature of the HCl + Mn2O7 reaction because of the highest oxidation state of Mn2O7.
Is HCl + Mn2O7 a redox reaction
HCl + Mn2O7 is a redox reaction because Mn acts as an oxidizer and increases the charge of Cl from -1 to 0, and HCl is a reducing agent as it reduces the charge of Mn from 7 to 2.
Is HCl + Mn2O7 a precipitation reaction
HCl + Mn2O7 is not a precipitation reaction since no precipitate formed during this reaction.
Is HCl + Mn2O7 reversible or irreversible reaction
HCl + Mn2O7 is considered an irreversible reaction because the products doesn’t undergo backward reaction.
Is HCl + Mn2O7 displacement reaction
HCl + Mn2O7 is a double displacement reaction because Mn displaces from Mn2O7 to MnCl2 and Cl displaced from HCl to Cl2.
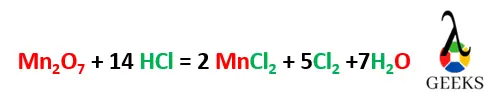
Conclusion
The HCl + Mn2O7 reaction is peculiar because of the oxidizing nature of Mn2O7, which looks thick green in color and can decompose at room temperature. HCl is used in textile and rubber industries and is mainly used to generate chlorides, affecting mucous membranes.
Read more facts on HCl:

Hi…I am Surya Satya Eluri. I have done my M.Sc in Organic Chemistry. I am very enthusiastic about the high-energy chemistry field. I love to write complicated chemistry concepts in understandable and simple words.
Let’s connect through LinkedIn: