In this article, we are going to discuss details on the reaction between HCl + H2O and also how to balance reactions with examples. HCl is one of the components of gastric juice
Hydrogen Chloride (HCl) gas which is highly soluble in water on dissolving in water forms Hydrochloric acid. The formula of hydrochloric acid is also HCl. Hydrochloric acid then dissociates in water and forms ions.
| HCl + H2O Type of reaction | Exothermic reaction(as heat is evolved) Reversible chemical reaction(as equilibrium is formed) Ionization Reaction (as ions are formed in the reaction) |
| Conjugate Acid | Has one hydrogen more than the base |
| Conjugate Base | Has one hydrogen less than the acid |
| Conjugate acid-base pairs in HCl + H2O | HCl and Cl– H3O+ and H2O |
| Type of ions formed in HCl + H2O reaction | H+(aq) or H3O+ and Cl–(aq) |
| In HCl + H2O reaction Acid is | HCl (Proton donor) |
| In HCl + H2O reaction Base is | H2O (Proton acceptor) |

What happens when HCl reacts with H2O?
When hydrogen chloride gas (HCl) reacts water, it forms hydrochloric acid.
Hydrochloric acid (also has formula HCl) thus formed, dissociates completely in water to form H+ and Cl– ions and large amount of heat is evolved in this reaction. Thus, this is an exothermic and ionization reaction
So, the reaction can be represented as –
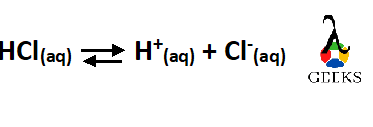
What type of reaction is HCl and H2O?
The reaction between HCl and H2O is an exothermic, reversible chemical reaction and an ionization reaction
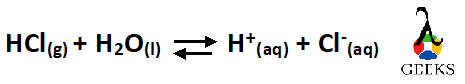
- Exothermic Reactions are those reactions in which heat is evolved and the temperature of the solution rises.
- Reversible chemical reactions are those reactions in which the reactants react to give products and products can react to give the reactants back. In short reversible reactions are those reactions which can be reversed.
- Ionization reactions is a chemical reaction in which a neutral atoms or molecules are converted into charged atoms or ions by give or take of electrons.
What is conjugate acid-base pair? Explain giving example of HCl + H2O reaction.
According to Bronsted and Lowry Theory –
An acid is the species which donates a proton(H+) and base is the species which accepts a proton(H+).
- The conjugate base is a substance formed when an acid loses a proton(H+) to a base.
- The conjugate acid is a substance formed when a base gains a proton(H+) from an acid.
Thus in short, an acid-base pair differs from each other either by the presence of a proton or by the absence of a proton.
For Example –

In the above example –
HCl and Cl– are the conjugate acid-base pairs and H2O and H3O+ are the conjugate acid-base pairs.
Is HCl and H2O an Exothermic or Endothermic reaction?
HCl + H2O is an exothermic reaction as large amount of heat is evolved in this reaction and temperature of the solution rises.
Write the net ionic Equation of HCl + H2O reaction.
The reaction HCl + H2O takes place as follows –
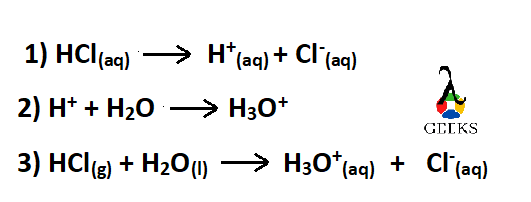
Equation 3) is the net ionic Equation of HCl + H2O reaction.
In the reaction HCl + H2O, which one is acting as acid and which is acting as base?
According to Bronsted-Lowry definition of acids and bases –
An acid is the species which donates a proton(H+)
A Base is the species which accepts a proton(H+).
Now our reaction is –
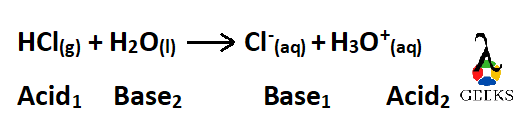
In the above equation, same subscript is used for each acid-base pair.According to Bronsted-Lowry theory, an acid is a species which donates a proton to a base so in the above equation –
- HCl is an acid which donates a proton to the base H2O and in doing so HCl gets converted to Cl–which is the conjugate base of HCl.
- H2O is a base which accepts a proton from HCl and in doing so H2O get converted into H3O+which is the conjugate acid of H2O
The conjugate base, Cl– is one hydrogen less than its acid HCl
The conjugate acid, H3O+ is one hydrogen more than its base H2O
How to balance the following Reaction?
Given reaction is-
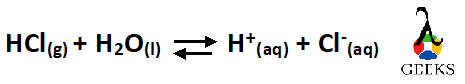
When Hydrogen Chloride gas reacts with water, it forms Hydrochloric acid which being a strong dissociates completely dissociates in water to form hydrogen ion and Chloride ion.
To balance this equation, we will count the atoms on both the reactant and the product sides-
| Element | Number of atoms in the Reactants (LHS) | Number of atoms in the Products (RHS) |
| Hydrogen | 3 | 3 |
| Chlorine | 1 | 1 |
| Oxygen | 1 | 1 |
The number of atoms at both the reactant and product side are the same so the reaction is a balanced one.
so the balanced Equation is –
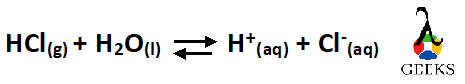
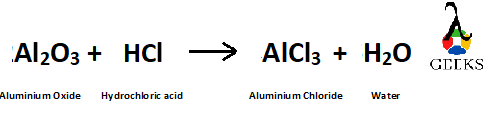
How to balance the reaction Al2O3 + HCl?
Al2O3 (Aluminium Oxide) reacts with dilute HCl (Hydrochloric acid) and produce AlCl3 (Aluminium Chloride) and H2O (water)
Given reaction is –
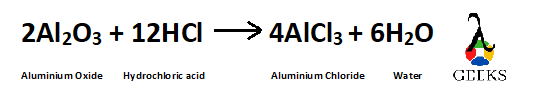
Balancing the reaction –
| Element | Number of atoms in the Reactants (LHS) | Number of atoms in the Products (RHS) |
| Aluminium | 2*2 = 4 | 1*4 = 4 |
| Oxygen | 3*2 = 6 | 1*6 = 6 |
| Hydrogen | 1*12=12 | 2*6 = 12 |
| Chlorine | 1*12=12 | 3*4 = 12 |
As all the atoms have become equal on both sides of the reaction so the reaction is now balanced.
Balanced reaction is –

How to balance the reaction COCl2 + HCl?
Cobalt(ll) Chloride reacts with hydrogen chloride and forms tetrachlorocobaltate(2-) and hydrogen gas.
Given reaction is –
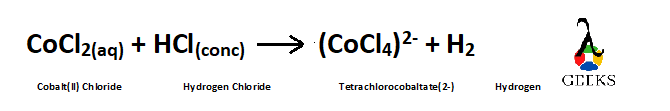
Balancing the Reaction –
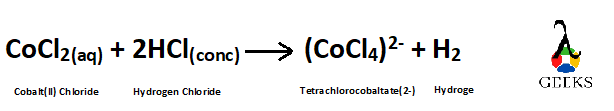
| Element | Number of atoms in the Reactants (LHS) | Number of atoms in the Products (RHS) |
| Cobalt | 1 | 1 |
| Chlorine | 2 + 1*2 = 4 | 4 |
| Hydrogen | 1*2=2 | 2 |
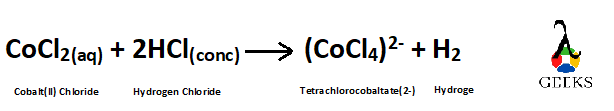
As all the atoms have become equal on both sides of the reaction so the reaction is now balanced.
Balanced reaction is –
Is HCl(l) + H2O(l) equal to HCl(aq)?
No, this is not the correct Equation. It should be written as –
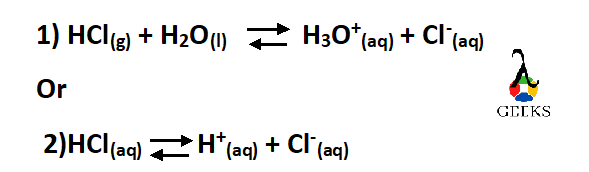
The term aqueous means a solution in which solvent is water, so when we are writing the phase of the substance as aqueous no need to write plus water (refer equation 2)
As Hydrogen Chloride (HCl) exists as a gas at STP that is at standard temperature and pressure so HCl is commonly stored as a concentrated aqueous solution that is Hydrochloric acid.
When HCl is dissolved in water, it dissociates completely into H+ and Cl–(aq). The H+ formed combines with water to form H3O+that is Hydronium ion.
Conclusion:
HCl + H2O is a reversible reaction. It is an exothermic reaction and large amount of heat is released in this reaction and Haq)and Cl–(aq) ions are formed.

Hi…I am Sonali Jham. I have done my Post-Graduation in Chemistry and also completed B. Ed. I am a Teacher and a Dietician by profession.
My hobby is reading and painting.
Let’s connect through LinkedIn-