Hydrochloric acid is an inorganic acid, and oxalic acid is an organic dicarboxylic acid. Let us study some facts about when these two acids are mixed.
Hydrochloric acid also called muriatic acid, is a highly reactive colorless acid with a molar mass of 36.458 g/mol. Oxalic acid is a white crystalline dicarboxylic acid with a molar mass of 126.065 g /mole in dihydrate form. Oxalic acid is a weak acid and has acid-base properties.
Let us look at some of the reaction characteristics of the HCl + H2C2O4 reaction.
What is the product of HCl and H2C2O4?
Oxalyl chloride (C2O2Cl2) is the main product obtained when oxalic acid reacts with hydrogen chloride.
- H2C2O4 + 2 HCl → C2O2Cl2 + 2 H2O
What type of reaction is HCl + H2C2O4?
The type of reaction observed between HCl and oxalic acid is an acid-base reaction, though HCl is a strong acid and H2C2O4 is a weak acid.
How to balance HCl + H2C2O4?
The process of balancing the general equation is given below with a step-by-step explanation.
Step 1: Find the number of atoms in reactants and products
The number of each of the atoms involved in the reactants and products is first determined. In accordance with the general equation, there are 3 H, 2 C, 4 O, and 1 Cl atoms present on the reactant side. On the products side, 2 H, 2 C, 3 O, and 2 Cl are present.
H2C2O4 + HCl → C2O2Cl2 + H2O
Step 2: Analyze the similarity between the number of molecules in reactants and products
The number of atoms of each element in the reaction is balanced by multiplying 2 with HCl on the reactant side and by 2 with H2O on the product side. The equation is now found to be balanced.
H2C2O4 + 2 HCl → C2O2Cl2 + 2 H2O
HCl + H2C2O4 Titration
Apparatus
Burette, burette stand, pipette, conical flask, conical flask.
Indicator
Phenolphthalein is used as the indicator in the acid-base titration. The endpoint for the titration is when the solution turns from pink to colourless.
Procedure
The strong acid HCl is taken in a beaker, and the base is taken in the burette. Near the equivalence point (endpoint), an acid-base indicator phenolphthalein having pH 8.2-10 is to be added. The endpoint of the titration is the disappearance of the pink colour.
HCl + H2C2O4 Net Ionic Equation
The net ionic equation cannot be written as the dissociation of oxalic acid is weak. Salts are formed by the addition of a strong acid to a weak acid. Oxalic acid is a weak acid, has a higher pH, and acts like a base.
HCl + H2C2O4 Conjugate Pair
- HCl and Cl – are conjugate acid-base pairs, while H2C2O4 and H3C2O4+ are another pair of conjugate acid-base.
- A conjugate acid-base pair is one in which the acid and the base differ by an H+ (proton).
HCl and H2C2O4 Intermolecular Forces
- The intermolecular forces that come into play in the HCl molecule are London dispersion forces and dipole-dipole interactive forces.
- Very strong Intermolecular hydrogen bonding exists in oxalic acid. Vander Waals dispersion forces and dipole-dipole interactions are also seen.
HCl + H2C2O4 Reaction Enthalpy
The reaction enthalpy is less negative as oxalic acid is a weak acid and dissociates only partially.
Is HCl + H2C2O4 a Buffer Solution?
The reaction of hydrochloric acid with oxalic acid does not produce an acidic buffer solution, as the former is a strong acid and the latter is a weak acid. Acidic buffer constitutes a weak acid with the salt of a weak acid and a strong base.
Is HCl + H2C2O4 a Complete Reaction?
The reaction between HCl and H2C2O4 is not a complete reaction. The reaction does not reach equilibrium as oxalic acid is a weak acid, and remains as molecules in solution.
Is HCl + H2C2O4 an Exothermic Reaction?
The between HCl and H2C2O4 is an exothermic reaction. Heat is released during the formation of the product, oxalyl chloride.
Is HCl + H2C2O4 a Redox Reaction?
The reaction between HCl and H2C2O4 is not a redox reaction as the oxidation state of the elements involved does not change during the reaction.
Is HCl + H2C2O4 a Precipitation Reaction?
HCl + H2C2O4 is considered as a precipitation reaction as insoluble oxalyl chloride is obtained.
Is HCl + H2C2O4 a Reversible or Irreversible Reaction?
The reaction between HCl and H2C2O4 is irreversible as oxalyl chloride which is formed is only slightly soluble in water.
Is HCl + H2C2O4 a Displacement Reaction?
The reaction HCl + H2C2O4 is a Double Displacement Reaction as the cations (H+) in HCl displace the anions (OH–) in oxalic acid to form two different compounds, namely, oxalyl chloride and water.
How to balance KMnO4 + H2C2O4 + HCl = KCl + MnCl2 + CO2 + H2O
The steps to balance the redox reaction of KMnO4 with H2C2O4 in an acidic medium are given below:
Step 1. Identification of oxidation and reduction half-reactions
Oxidation half-reaction:
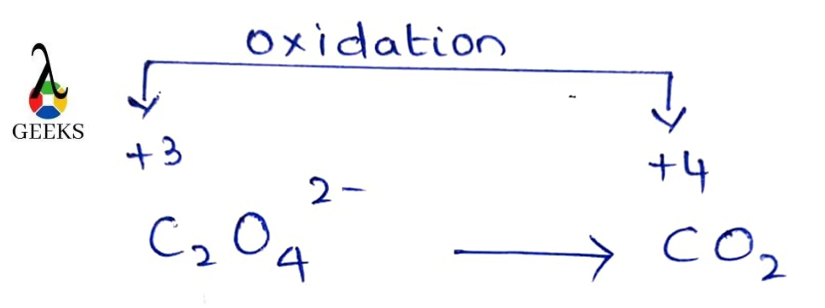
Oxidation half-reaction
Reduction half-reaction:

Reduction half-reaction
Step 2. Balance all atoms other than oxygen
C2O42-→ 2CO2
Step 3. Balance oxygen and the number of electrons
The number of oxygen atoms is balanced by adding H2O on the side deficient of O and doubling the number of H+ ions on the opposite side. Then balance electrons on both sides.
- C2O42-→ 2CO2 + 2e–
- MnO41- +8 H+ + 5e–→ Mn2+ + 4 H2O
Step 4. Equalize the number of electrons on both sides of the reaction
In any reaction, the Gain of electrons = Loss of electrons. So, multiply the oxidation and reduction half-reactions obtained in Step 3 by 5 & 2, respectively. Then, add the two equations.
5C2O42- + 2MnO41- + 16H+ + 10e– → 2Mn2+ 10CO2 + 8H2O+ 10e–
Step 5. Finalize the net balanced equation
Cancel the electrons on either side of the reaction to get the net balanced equation.
2KMnO4+5H2C2O4+6HCl = 2KCl+2MnCl2+10CO2+8H2O
Conclusion
The addition of HCl to H2C2O4 results in the conversion of some of the oxalic acid to oxalyl chloride. Here, H2C2O4 behaves like a basic solvent.
Read more facts on HCl:

Hi Everyone. I am Vishnupriya T. I have pursued a Master’s in Chemistry and a PG Diploma in Quality Management. Currently working as an SME in Chemistry, aiming to enrich my curiosity and knowledge. I would love to share knowledge and connect with others at my LinkedIn profile: