HCl is a strong acid while FeCO3 is a base. Let us explore the reaction between HCl and FeCO3 in detail.
Hydrochloric acid (HCl) is a colorless, transparent liquid. It is a strong acid with a pungent odor. FeCO3 is iron(ll) Carbonate. It exists as white powder or crystals. It is a base.
Let us discuss the products formed in this reaction, type of reaction, balancing the reaction, net ionic equation, titration, conjugate pairs, reaction enthalpy and some more important in this article.
What is the product of HCl and FeCO3?
The product formed in the reaction of HCl and FeCO3 are ferrous chloride (FeCl2), carbon dioxide (CO2) and water (H2O) are as below.
2HCl + FeCO3 –> FeCl2 + CO2 + H2O
What type of reaction is HCl + FeCO3?
HCl + FeCO3 is a acid-base reaction or a neutralization reaction where acid and base react to form salt and water. It is also displacement reaction where there is an exchange of ions between the reacting species.
How to balance HCl + FeCO3?
To balance the chemical reaction, the following steps need to be followed-
Step 1: Write the skeletal chemical equation
Write the unbalanced chemical equation with reactants on the right side, left pointing arrow and products on the left side of the reaction.
HCl + FeCO3 –>FeCl2 + CO2 + H2O
Step 2: Count the number of moles for each element
Count the number of moles for each element on the reactant and the product side in the chemical Equation.
| Elements | No. of moles on Reactant side | No. of moles on Product side |
|---|---|---|
| H | 1 | 2 |
| Cl | 1 | 2 |
| Fe | 1 | 1 |
| C | 1 | 1 |
| O | 3 | 3 |
- As we can see from the above table that H and Cl are the only unbalanced atoms. Adding stoichiometric coefficient before the appropriate molecule will balance H and Cl.
- In this reaction HCl molecule contains both H and Cl atom. Multiply HCl by 2 will balance the whole chemical equation.
Step 3: Write the balanced chemical equation
2HCl + FeCO3 –>FeCl2 + CO2 + H2O
HCl and FeCO3 titration
To determine the % composition of FeCO3 in a given sample, we need to titrate it with HCl solution. The titration between HCl and FeCO3 can be carried out as follows-
Apparatus
Burette stand, Graduated Burette, volumetric flask, pipette, beakers
Chemicals Needed
FeCO3 (whose concentration needs to be analysed), HCl (concentration is known), Indicator
Procedure
- First the solution of HCl needs to be standardized. To standardize the HCl solution, titrate the solution of HCl with a primary standard like Na2CO3.
- Write the balanced neutralization reaction of HCl with Na2CO3. Take measured amount of Na2CO3 dissolve it in some water. Add a colorimetric indicator to it.
- HCl solution was added to Na2CO3 to reach the equivalence point.
- Now, the amount of FeCO3 can be determined by titrating it with the standardized HCl solution.
- The amount of HCl required to neutralise the FeCO3, will give the amount of FeCO3 in the given sample.
HCl + FeCO3 net ionic equation
Complete ionic equation can be written as follows –
2H+(aq) + 2Cl–(aq) + FeCO3(s) = 2H+(aq) + CO32- (aq) + Fe2+ (aq) + 2Cl– (aq)
- Now, to write net ionic equation we will remove the spectator ions from the equation. Net ionic equation will consist of the species which are taking part in the reaction.
- The net ionic equation is as follows-
FeCO3(s) = Fe2+ (aq) + CO32- (aq)
HCl and FeCO3 conjugate pairs
HCl being a strong acid donates a proton(H+) and forms Cl– ion. CO32- being a weak base accepts the proton to form its conjugate acid HCO3–. The conjugate pairs of HCl + FeCO3 is as follows-

HCl and FeCO3 intermolecular forces
The intermolecular forces present in HCl and FeCO3 are as under –
- The intermolecular forces present in HCl are stronger Dipole-dipole forces and London dispersion forces. The dipole -dipole forces arise due to the as Cl is more electronegative than H atom.
- FeCO3 is an ionic compound so the intermolecular force present is electrostatic force. Thus, there is a strong force of attraction between Fe2+and CO32-.
HCl and FeCO3 reaction enthalpy
The reaction enthalpy for the reaction of HCl and FeCO3 is 81.87 kJ/mol at 298 K. The reaction enthalpy can be calculated by using the formula –
∆H0f = ∆H0f (products) – ∆H0f (reactants)
| Compounds | ∆H0f (kJ/mol) |
|---|---|
| HCl | -334.32 |
| FeCO3 | -738.15 |
| FeCl2 | -311.34 |
| CO2 | -393.5 |
| H2O | -285.76 |
Is HCl and FeCO3 a buffer solution?
HCl and FeCO3 is not a buffer solution. A buffer solution is a water-based solution of a weak acid and its conjugate base or a weak base and its conjugate acid. In this reaction, HCl is a strong acid while FeCO3 is a base. So HCl and FeCO3 is not a buffer.
Is HCl and FeCO3 a complete reaction?
HCl and FeCO3 is a complete reaction because it products formed do not show the formation of products.
Is HCl and FeCO3 an exothermic or endothermic reaction?
The reaction between HCl and FeCO3 is an endothermic reaction because reaction enthalpy of this reaction is positive. Positive value of enthalpy means the reaction is non-spontaneous.
Is HCl and FeCO3 a redox reaction?
HCl and FeCO3 does not involve electron transfer so it is not a redox reaction. It is a acid-base reaction.
Is HCl and FeCO3 a precipitation reaction?
HCl and FeCO3 is not a precipitation reaction because the products formed are not insoluble in water. FeCl2 is soluble in water. CO2 is a gas which releases from the reaction medium.
Is HCl and FeCO3 reversible or irreversible reaction?
HCl and FeCO3 reaction is irreversible because the gas (CO2) formed in the reaction increases the entropy of the reaction. It is not possible to collect the gas molecules and turn them back into reactants.
Is HCl and FeCO3 displacement reaction?
HCl and FeCO3 is a double displacement reaction because in this reaction there is a exchange of ions CO32- and Cl– between the reacting species and there is also evolution of CO2 gas.
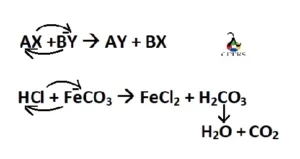
Conclusion:
HCl and FeCO3 is an acid-base (neutralization) reaction. It is an endothermic reaction. The reaction enthalpy for the reaction is found to be 81.87 kJ/mol.

Hi…I am Sonali Jham. I have done my Post-Graduation in Chemistry and also completed B. Ed. I am a Teacher and a Dietician by profession.
My hobby is reading and painting.
Let’s connect through LinkedIn-