NaH2PO4 is a basic salt of sodium produced from NaOH or NaHCO3 and it can easily react with HBr. Let us explore the mechanism of the reaction between HBr and NaH2PO4.
NaH2PO4 or monosodium phosphate is a basic salt that can be prepared from sodium phosphate where two sodium replaced by two H atoms. In NaH2PO4, central P is attached with one double-bonded O, two -OH, and one -O with a single bond. In HBr, there is a covalent bond present between H and Br.
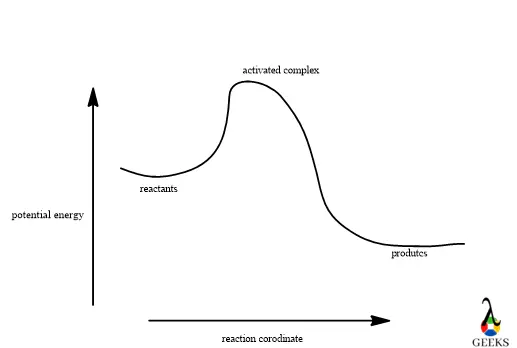
Although there will be some parameters and limitations present in this reaction. Now we can discuss more the mechanism of the reaction like enthalpy, redox reaction, intermolecular force, conjugate pairs, etc with an explanation in the following part of the article.
1. What is the product of HBr and NaH2PO4?
Sodium bromide and phosphoric acid are formed as major products when HBr and NaH2PO4 were reacted together.
HBr + NaH2PO4 = NaBr + H3PO4
2. What type of reaction is HBr + NaH2PO4?
HBr + NaH2PO4 reaction is an example of a double displacement reaction, and a redox and precipitation reaction. It is also a salt metathesis reaction along with an acid production reaction.
3. How to balance HBr + NaH2PO4?
HBr + NaH2PO4 = NaBr + H3PO4, we have to balance the equation in the following way-
- First, we label all the reactants and products by A, B, C, and D as there are four different molecules obtained for this reaction and the reaction looks like this,
- A HBr + B NaH2PO4 = C NaBr + D H3PO4
- Equating the coefficients for the same type of elements by rearranging them.
- After the rearrangement of coefficients of the same elements by their stoichiometric proportion, we get,
- H = A = 2B = 2C = D, Br = A = C, Na = B = C, P = B = D, O = 4B = 4D
- Using the Gaussian elimination and equating all the equations we get, A = 1, B = 1, C = 1, and D = 1
- The overall balanced equation will be,
- HBr + NaH2PO4 = NaBr + H3PO4
4. HBr + NaH2PO4 titration
To estimate the quantity of phosphate or bromide we can perform a titration between NaH2PO4 and HBr.
Apparatus used
We need a burette, conical flask, burette holder, volumetric flask, and beakers for this titration.
Titre and titrant
HBr versus NaH2PO4, HBr acts as a titrant taken in the burette and the molecule to be analyzed is NaH2PO4 taken in a conical flask.
Indicator
The whole titration is done in an acidic medium or acidic pH so the best suitable indicator will be phenolphthalein which gives perfect results for this titration at given pH.
Procedure
The burette is filled with standardized HBr. NaH2PO4 is taken in a conical flask along with respective indicators. HBr is added dropwise to the conical flask and the flask is shaken constantly. After a certain time, when the endpoint arrives, the indicator changes its color and the reaction is done.
We repeat the titration several times for better results and then we estimate the bromide or phosphate quantity by the formula V1S1 = V2S2.
5. HBr+ NaH2PO4 net ionic equation
The net ionic equation between HBr + NaH2PO4 is as follows,
H+(aq.) + Br–(aq.) + Na+(aq.) + 2H+(aq.) + PO43-(aq.) = Na+(aq.) + Br–(aq.) + 3H+(l) + PO43-(l)
To derive the net ionic equation, the following steps are required,
- HBr will be ionized as proton and bromide as it is strong acid and electrolyte.
- After that NaH2PO4 also dissociates into Na+ and H+ ions and PO4– as counter ion as it is also a strong electrolyte
- In the product part, NaBr is ionized into Na+ and Br–as it is a strong electrolyte and salt.
- After that phosphoric acid can be ionized into three protons and phosphate ions as it is a strong acid and the dissociation constant is low.
6. HBr+ NaH2PO4 conjugate pairs
HBr+ NaH2PO4 reaction has the following pairs,
- Conjugate pair of HBr = Br–
- Conjugate pair of PO43- = HPO42-
- Conjugate pair of HPO42- = H2PO4–
- Conjugate pair of H2PO4– = H3PO4
7. HBr and NaH2PO4 intermolecular forces
HBr+ NaH2PO4 reaction has the following intermolecular forces,
- The intermolecular force present in HBr is the strong electrostatic force between protons and bromide ions.
- In NaH2PO4 there are electronic interactions and coulumbic force present.
- In NaBr ionic interaction is present and for phosphoric acid H-bonding is present.
| Molecule | Acting force |
| HBr | Electrostatic, van der waal’s Dipole interaction |
| NaH2PO4 | Strong electrostatic force and ionic interaction, Coulumbic force |
| NaBr | Electrostatic force, ionic interaction, |
| H3PO4 | Covalent force, H-bonding |
8. HBr + NaH2PO4 reaction enthalpy
HBr + NaH2PO4 reaction enthalpy is -1227.66 KJ/mol which can be obtained by the formula: enthalpy of products – enthalpy of reactants.
| Molecule | Enthalpy (KJ/mol) |
| NaH2PO4 | -369 |
| HBr | -36.45 |
| NaBr | -361.41 |
| H3PO4 | -1271.7 |
and Products
9. Is HBr + NaH2PO4 a buffer solution?
HBr + NaH2PO4, reaction formed the buffer solution of the mixture of H3PO4 and NaBr can control the pH even after adding a base.
10. Is HBr + NaH2PO4 a complete reaction?
HBr + NaH2PO4 reaction is complete because it gives two major ones: an electrolytic salt and another phosphoric acid.
11. Is HBr + NaH2PO4 an exothermic or endothermic reaction?
HBr + NaH2PO4 reaction is exothermic in terms of thermodynamics first law. This reaction released more energy and temperature to the surroundings, where δH is always negative.
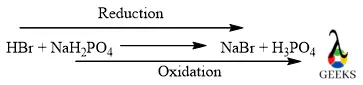
12. Is HBr + NaH2PO4 a redox reaction?
HBr + NaH2PO4 reaction is a redox reaction because in this reaction bromine gets reduced and also phosphorus gets oxidized. Where HBr acts as a reducing agent and NaH2PO4 acts as an oxidizing agent.
HBr and NaH2PO4 Reaction
13. Is HBr + NaH2PO4 a precipitation reaction
HBr + NaH2PO4 reaction is a precipitation reaction because phosphoric acid gets precipitated in the solution at certain pH.
14. Is HBr + NaH2PO4 reversible or irreversible reaction?
HBr+ NaH2PO4 reaction is irreversible because it produced acid. Due to the production of the acid molecule the equilibrium shifts towards the right-hand side only or forward directions.
HBr + NaH2PO4 —-> NaBr + H3PO4
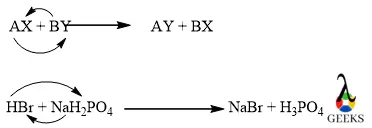
15. Is HBr + NaH2PO4 displacement reaction?
The reaction between HBr + NaH2PO4 is an example of a double displacement reaction. Because in the above reaction H+ was displaced by Na+ from HBr and Na is also displaced by H+ and attached to Br.
Conclusion
The reaction between HBr and NaH2PO4 is important because it can produce phosphoric acid. So, the reaction is very important in the industrial aspect. The reaction is also important for the production of sodium bromide salt.

Hi……I am Biswarup Chandra Dey, I have completed my Master’s in Chemistry from the Central University of Punjab. My area of specialization is Inorganic Chemistry. Chemistry is not all about reading line by line and memorizing, it is a concept to understand in an easy way and here I am sharing with you the concept about chemistry which I learn because knowledge is worth to share it.