Mn is a transition metal with having shiny grey appearance. It is hard and brittle and found in combination with iron. Let us read how Mn reacts with HBr through this article.
Manganese (Mn) and Hydrogen bromide (HBr) react to form salt and hydrogen gas. Mn belongs to d-block elements in the periodic table. The atomic number of Mn is 25 and has a half-filled stable electronic configuration. HBr is a strong acid composed of hydrogen and boron. It normally exits as a colourless gas.
We will discuss key facts about the HBr+Mn reaction, like the products, type of reaction, balanced chemical equation and conjugate pairs in the article below.
What is the product of HBr and Mn
Manganese bromide (MnBr2) and hydrogen (H2) gas are the products of the HBr + Mn reaction. The chemical equation for this reaction is,
Mn + HBr = MnBr2 + H2
What type of reaction is HBr + Mn
HBr + Mn reaction is a redox reaction in which metal reacts with an acid it gets consumed completely to form salt and release hydrogen gas.
How to balance HBr + Mn
The balanced chemical equation for the HBr + Mn reaction is,
Mn + 2HBr = MnBr2 + H2
The steps involved in deriving the balanced chemical equation are as follows:
- Noted the unbalanced chemical equation, Mn + HBr = MnBr2 + H2
- The number of moles of atoms available on both sides of thet chemical equation is;
| Atoms | Number on reactant side | Number on product side |
|---|---|---|
| H | 1 | 2 |
| Mn | 1 | 1 |
| Br | 1 | 2 |
- As number of hydrogen and boron are not balanced. Therefore, we multiply a coefficient of 2 with HBr to balance the equation.
- Thus, the balanced chemical equation for Mn + HBr reaction is,
- Mn + 2HBr = MnBr2 + H2
HBr + Mn titration
Titration between HBr and Mn is not feasible as Mn is a metal that cannot be categorised in any titration.
HBr + Mn net ionic equation
HBr + Mn net ionic equation is,
Mn (s) + 2H+ (aq.) + 2Br– = MnBr2 (aq.) + H2 (g)
The steps involved during the derivation of the net ionic equation are:
- Noted the general balanced chemical equation for the reaction.
- Mn + 2HBr = MnBr2 + H2
- Indicated the chemical state (s, l, g and aq) of each compound.
- Mn (s) + 2HBr (aq.) = MnBr2 (aq.) + H2 (g)
- Divided the electrolytes which are strong enough to break into their corresponding ions in an aqueous solution.
- Mn (s) + 2H+ (aq.) + 2Br– (aq.) = MnBr2 (aq.) + H2 (g)
- Eliminated the ions available on both sides of the chemical equation to obtain the net ionic equation.
- Mn (s) + 2H+ (aq.) + 2Br– (aq.) = MnBr2 (aq.) + H2 (g)
HBr + Mn conjugate pairs
The HBr and Mn do not have any conjugate pair as no conjugate acid or base is made throughout the process.
HBr and Mn intermolecular forces
- The intermolecular force of attraction present in HBr molecules is dipole-dipole interaction.
- Metallic bonds are present in Mn metal.
- H2 contains London dispersion forces.
HBr + Mn reaction enthalpy
The HBr + Mn reaction enthalpy is -141.7 kJ/mol. The standard enthalpy of formation for the reactants involved in chemical equations is:
| Compounds | Enthalpy of reaction (in kJ/mol) |
|---|---|
| HBr | -121.6 |
| Mn | 0 |
| MnBr2 | -384.9 |
| H2 | 0 |
Reaction Enthalpy ΔHf = Standard enthalpy of products – Standard enthalpy of reactants
Thus, ΔHf = (-384.9 – 0) – (-2*(121.6) – 0))
ΔHf = -141.7 kJ/mol.
Is HBr + Mn a buffer solution
The HBr + Mn combination does not yield a buffer solution because HBr is a strong acid and there must be a weak acid to form the buffer solution.
Is HBr + Mn a complete reaction
HBr + Mn reaction is a complete reaction as no other steps can be performed.
Is HBr + Mn an exothermic or endothermic reaction
HBr + Mn reaction is an exothermic reaction because the enthalpy of reaction has a value of -141.7 kJ/mol which is negative.
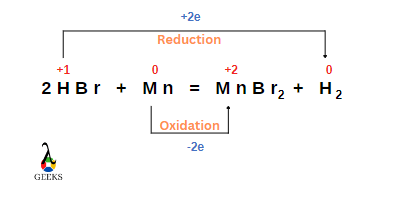
Is HBr + Mn a redox reaction
HBr + Mn reaction is a redox reaction in which hydrogen gets reduced and Mn gets oxidised.
Is HBr + Mn a precipitation reaction
HBr + Mn reaction is not a precipitation reaction as no solid matter is produced at the end of the reaction.
Is HBr + Mn reversible or irreversible reaction
HBr + Mn reaction is an irreversible reaction as the H2 gas evolved during the process cannot be reverted into the reaction mixture and the chemical path for this reaction has only one way of proceeding.
Is HBr + Mn displacement reaction
HBr + Mn reaction is a single displacement reaction in which Mn displaces H from HBr to form the corresponding salt.
Conclusion:
This article concludes that HBr completely dissolves metals that are provided in it to form salt precipitates and release hydrogen gas when taken in its aqueous form. The H2 gas evolution can be checked using a burning candle. Mn is initially found combined with iron and needs to be separated.

Hi, I am Sahil Singh. I completed my graduation in Bachelor of Science. I always have keen interest in Physics & Chemistry. I worked on my own blog for 1 year in the technology and gaming niche. I try my best to provide valuable knowledge through my articles.
You can reach out to me on LinkedIn: