HBr is strong inorganic acid that can easily react with electron-deficient Mg2Si without a catalyst. Let us predict the mechanism behind the reaction.
Mg2Si or magnesium silicide is a lewis base because the silicon center has a vacant d orbital that is energetically accessible and can accept an electron. It is also used as an electrophilic center because the charge density over Mg is being dragged away toward the electronegative Si site. HBr is strong acid it can easily release protons.
The reaction between HBr and Mg2Si does not require any catalyst or temperature and pressure because one is more reactive. Now we can discuss more the mechanism of the reaction like enthalpy, redox reaction, intermolecular force, conjugate pairs, etc with an explanation in the following part of the article.
1. What is the product of HBr and Mg2Si?
Magnesium bromide and silane are formed as major products when HBr and Mg2Si are subject to react together.
HBr + Mg2Si = MgBr2 + SiH4
2. What type of reaction is HBr + Mg2Si?
HBr + Mg2Si reaction is an example of a double displacement reaction, and a redox and precipitation reaction. It is also a nucleophile and electrophile reaction because the former is a nucleophile and the latter is an electrophile.
3. How to balance HBr + Mg2Si?
HBr + Mg2Si = MgBr2 + SiH4, we have to balance the equation in the following way-
- First, we label all the reactants and products by A, B, C, and D as there are four different molecules obtained for this reaction and the reaction looks like this,
- A HBr + B Mg2Si = C MgBr2 + D SiH4
- Equating the coefficients for the same type of elements by rearranging them.
- After the rearrangement of coefficients of the same elements by their stoichiometric proportion, we get,
- H = A = D, Br = A = 2C, Mg = 2B = C, Si = B = D
- Using the Gaussian elimination and equating all the equations we get, A = 4, B = 1, C = 3, and D = 1
- The overall balanced equation will be,
- 4HBr + Mg2Si = 2MgBr2 + SiH4
4. HBr + Mg2Si titration
To estimate the quantity of magnesium or strength of acid we can perform a titration between Mg2Si and HBr.
Apparatus used
We need a burette, conical flask, burette holder, volumetric flask, and beakers for this titration.
Titre and titrant
HBr versus Mg2Si, HBr acts as a titrant taken in the burette and the molecule to be analyzed is Mg2Si taken in a conical flask.
Indicator
The whole titration is done in an acidic medium or acidic pH so the best suitable indicator will be phenolphthalein which gives perfect results for this titration at given pH.
Procedure
The burette is filled with standardized HBr. Mg2Si is taken in a conical flask in solution along with respective indicators. HBr is added dropwise to the conical flask and the flask is shaken constantly. After a certain time, when the endpoint arrives, the indicator changes its color and the reaction is done.
5. HBr+ Mg2Si net ionic equation
The net ionic equation between HBr + Mg2Si is as follows,
H+(aq.) + Br–(aq.) + 2Mg2+(aq.) + Si4-(aq.) = Mg2+(aq.) + Br–(aq.) + SiH4(g)
- HBr will be ionized as proton and bromide as it is strong acid and electrolyte.
- After that Mg2Si also dissociates into Mg2+ ion and Si4- ion as it is also a strong electrolyte
- In the product part, MgBr2 is ionized into Mg2+ and Br–as it is a strong electrolyte and salt.
- SiH4 exists in the gaseous state so it can be dissociated.
6. HBr+ Mg2Si conjugate pairs
In the reaction, HBr+ Mg2Si conjugate pairs will be the corresponding de-protonated and protonated form of that particular species which are listed below-
- Conjugate pair of HBr = Br–
- Conjugate pair of SiH4 = SiH5+ (super acid)
7. HBr and Mg2Si intermolecular forces
HBr+ Mg2Si reaction has the following intermolecular forces,
- The intermolecular force present in HBr is the strong electrostatic force between protons and bromide ions.
- In Mg2Si there are electronic interactions and coulumbic force present.
- In MgBr2 ionic interaction is present and for SiH4 van der waal’s force along with London dispersion force is present.
| Molecule | Acting force |
| HBr | Electrostatic, van der waal’s Dipole interaction |
| Mg2Si | Strong electrostatic force and ionic interaction, Coulumbic force |
| MgBr2 | Electrostatic force, ionic interaction, |
| SiH4 | Covalent force, London dispersion force |
8. HBr + Mg2Si reaction enthalpy
HBr + Mg2Si reaction enthalpy is -1176.78 KJ/mol which can be obtained by the formula: enthalpy of products – enthalpy of reactants.
| Molecule | Enthalpy (KJ/mol) |
| Mg2Si | -16.69 |
| HBr | -36.45 |
| MgBr2 | -524.3 |
| SiH4 | 34.31 |
and Products
9. Is HBr + Mg2Si a buffer solution?
In the reaction between HBr + Mg2Si, there is no such buffer formed but the mixture of MgBr2 and SiH4 can control the pH.
10. Is HBr + Mg2Si a complete reaction?
The reaction between HBr + Mg2Si is complete because it gives one major product: an electrolytic salt and other silane gas as a by-product.
11. Is HBr + Mg2Si an exothermic or endothermic reaction?
The reaction of HBr + Mg2Si is exothermic in terms of thermodynamics first law. This reaction released more energy and temperature to the surroundings, where δH is always negative.
12. Is HBr + Mg2Si a redox reaction?
HBr + Mg2Si reaction is a redox reaction because in this reaction Si gets reduced and Br gets oxidized. Where HBr acts as a reducing agent and Mg2Si acts as an oxidizing agent.
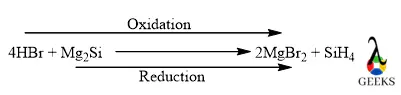
HBr and Mg2Si Reaction
13. Is HBr + Mg2Si a precipitation reaction
The reaction between HBr + Mg2Si is a precipitation reaction because Br2 gets precipitated in the solution at certain pH.
14. Is HBr + Mg2Si reversible or irreversible reaction?
The reaction between HBr+ Mg2Si is irreversible because it produced silane gas. Due to the production of the gaseous molecule the entropy of the reaction increases. Therefore, equilibrium shifts towards the right-hand side only or forward directions.
4HBr + Mg2Si —-> 2MgBr2 + SiH4(g)
15. Is HBr + Mg2Si displacement reaction?
The reaction between HBr+ Mg2Si is an example of a double displacement reaction. Because in the above reaction Br- was displaced by Mg+ from HBr and Si gets displaced by H+ from Mg2Si.
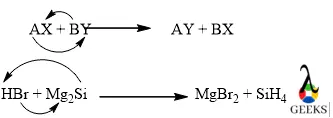
Conclusion
The reaction between HBr and Mg2Si is important because it can produce Silane gas. So, we have to take more cautious when the reaction is going on. It is an industrially important reaction for the production of MgBr2 salt.

Hi……I am Biswarup Chandra Dey, I have completed my Master’s in Chemistry from the Central University of Punjab. My area of specialization is Inorganic Chemistry. Chemistry is not all about reading line by line and memorizing, it is a concept to understand in an easy way and here I am sharing with you the concept about chemistry which I learn because knowledge is worth to share it.