Mg is an alkaline earth metal having an atomic number of 12. Let us discuss the products obtained from the reaction between the reaction of HBr and Mg.
HBr is one of the strongest acids and also a strong oxidizing agent. Magnesium is a shiny grey solid material with the mass number of 24. This metal is placed in the 2nd group and 3rd period of the periodic table.
This article explores the products, balancing method, change of enthalpy, type, reversibility, and many more relevant facts of the reaction between HBr + Mg in detail.
What is the product of HBr and Mg?
Magnesium bromide (MgBr2) crystals and hydrogen gas (H2) are obtained as the products when magnesium metal reacts with hydrobromic acid (HBr).
Mg (s) + 2HBr (aq) = MgBr2 (aq) + H2 (g)
What type of reaction is HBr + Mg?
The reaction HBr+ Mg is one type of-
How to balance HBr+ Mg?
To balance the reaction of HBr + Mg, these following steps should be followed-
- The unbalanced chemical equation is written first using a right arrow sign to signify that the reaction is not balanced yet. Mg (s) + HBr (aq) ➝ MgBr2 (aq) + H2 (g)
- The mole numbers of each of the reacting elements Mg, H, and Br) should be determined on both the reactant and product sides.
| Elements | Mole numbers on the reactant side | Mole numbers on the product side |
| Mg | 1 | 1 |
| H | 1 | 2 |
| Br | 1 | 2 |
- To balance both sides (reactant and product) we have to multiply 2 with the mole number of HBr on the reactant side.
- Therefore, the final and the balanced equation will be- Mg (s) + 2HBr (aq) = MgBr2 (aq) + H2 (g)
HBr+ Mg Net Ionic Equation
The net ionic equation of the reaction of HBr+ Mg is-
Mg0 (s) + 2H+ (aq) + 2Br– (aq) = Mg2+ (aq) + 2Br– (aq) + H2 (g)
HBr+ Mg Conjugate Pairs
The conjugate pairs (pair of compounds differ by one proton) of the reaction HBr + Mg is –
- The conjugate pair of HBr is Br–
- There is no existence of any conjugate pair for MgBr2, Mg, and H2.
HBr + Mg Titration
The titration between HBr+Mg cannot be performed because a gas (H2) is evolving from the reaction medium, and metal cannot directly participate in a redox titration. Redox titrations are only done to evaluate the amount of metal in any metal compound. But here, metal is already present along with strong acid.
HBr + Mg Intermolecular Forces
The intermolecular forces present in HBr + Mg are-
- HBr is a polar covalent acid. Therefore, dipole-dipole forces and London dispersion forces are present in HBr as intermolecular forces. It also participates in intermolecular hydrogen bonding with other HBr molecules.
- Mg is a single metal atom. Thus, there are no intermolecular forces present in Mg.
- MgBr2 is an ionic compound. A strong electrostatic attraction force is present between Mg2+ and Br– ion.
- H2 is a nonpolar covalent molecule. Thus, only the London dispersion force is present between H2 molecules.
HBr + Mg Reaction Enthalpy
The enthalpy change of the reaction HBr + Mg is -466.85 KJ/mol. This value is obtained from the following formation enthalpy values and the mathematical calculation.
| Elements | Formation enthalpy |
| Mg | 0 KJ/mol |
| HBr | -121.55 KJ/mol |
| MgBr2 | -709.95 KJ/mol |
| H2 | 0 KJ/mol |
- Enthalpy of the reaction = enthalpy of the products – enthalpy of the reactants
- The enthalpy of the reaction between HBr + Mg is = {(ΔHMgBr2 + ΔHH2) – (ΔHMg + ΔHHBr)} = [( -709.95 + 0) – {(-121.55 × 2) + 0}] KJ/mol = -466.85 KJ/mol
Is HBr + Mg a buffer solution?
HCl + Mg is not a buffer solution because it is neither a mixture of a weak acid and its conjugate base (CH3COOH and CH3COONa, acidic buffer) nor a weak base and its conjugate acid (NH4OH and NH4Cl, basic buffer). It is a reaction between a metal and a strong acid and this mixture cannot act as a buffer solution.
Is HBr + Mg a complete reaction?
HBr + Mg can be a complete reaction if the desired products (MgBr2 and H2O) are written along with the reactants. HBr and Mg are only the reactants of this reaction. Therefore, writing only the reactant or only the products is not the correct way to express a complete chemical reaction. The complete reaction is-
Mg (s) + 2HBr (aq) = MgBr2 (aq) + H2 (g)
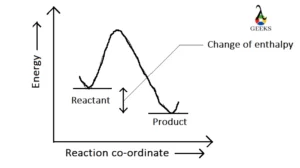
Is HBr + Mg an exothermic or endothermic reaction?
HBr+Mg is an exothermic reaction because the change of enthalpy is negative (-466.85 KJ/mol) in this reaction. This negative value signifies that heat is absorbed on the product side and generated on the reactant side and the reaction in the forward direction (product side) will be favored at low temperatures.
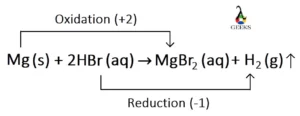
Is HBr + Mg a redox reaction?
HBr + Mg is a redox reaction because the oxidation state of all the elements is changed from the reactant side to the product side. In this reaction, Mg acts as the reducing agent and it reduces hydrogen to form gaseous H2. On the other side, HBr works as the oxidizing agent, and it oxidizes Mg to form MgBr2.
| Elements | Oxidation state on the reactant side | Oxidation state on the product side |
| Mg | 0 | +2 |
| H | +1 | 0 |
| Br | -1 | -1 |
Is HBr + Mg a precipitation reaction?
HBr + Mg is not a precipitation reaction because none of the products (MgBr2 and H2) appears as precipitate after the completion of the reaction. MgBr2 is soluble in the solution and gaseous hydrogen evolves as bubbles from the reaction medium.
Is HBr + Mg a reversible or irreversible reaction?
HBr + Mg is an irreversible reaction because the product side is more stable than the reactant side due to forming a gaseous product. Gas always possesses a large amount of entropy than a liquid or solid. Due to this entropy factor, the reaction will proceed in the forward direction.
Is HBr + Mg displacement reaction?
HBr + Mg is an example of a single-displacement reaction. In this reaction, Mg replaces hydrogen from HBr to form magnesium bromide (MgBr2). It is called single displacement because only one element (hydrogen) is displaced by Mg to form the products.
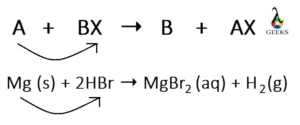
Conclusion
HBr is mainly used to manufacture inorganic bromides (ZnBr2, CaBr2 NaBr, etc) and organobromine compounds. It is also used for the catalysis of many alkylation reactions, the cleaving of ethers, and the extraction of certain ores. Mg is applied in car seats, laptops, luggage, power tools, etc.

Hello,
I am Aditi Ray, a chemistry SME on this platform. I have completed graduation in Chemistry from the University of Calcutta and post graduation from Techno India University with a specialization in Inorganic Chemistry. I am very happy to be a part of the Lambdageeks family and I would like to explain the subject in a simplistic way.
Let’s connect through LinkedIn-https://www.linkedin.com/in/aditi-ray-a7a946202