The non-stoichiometric iron complex FeS can react with a variety of acids, including HBr, and is water soluble. Lets us observe how both of them reacts.
When iron reacts with a potent acid like HBr, it changes its oxidation state from being present at a +2 to a state that allows it to be further oxidized by a suitable reagent. Most iron-sulfur proteins, including the protein cluster Rubredoxin, contain FeS and are used in one-electron transfer processes.
We will go over important and relevant information regarding the HBr + FeS reaction, such as the redox reaction, reaction type, products, and balanced chemical equation.
What is the product of HBr and FeS ?
Hydrogen Sulphide H2S and Ferrous Bromide FeBr2 are formed when Hydrobromic acid HBr reacts with Iron Sulphide FeS.
FeS + HBr = H2S + FeBr2
What type of reaction is HBr + FeS
HBr + FeS is a type of Precipitation Reaction, Acid-Base Reaction, Redox Reaction and Displacement Reaction.
How to balance HBr + FeS
The following steps need to be followed while balancing a chemical equation.
- Given below is an unbalanced chemical reaction of HBr and FeS,
FeS + HBr = H2S + FeBr2
- Record the moles of each element on the reactant and product sides.
| Element | Reactant | Product |
| Fe | 1 | 1 |
| S | 1 | 1 |
| H | 1 | 2 |
| Br | 1 | 2 |
- Now, the number of moles present on each side of the reactant and product must be equal in order to balance the chemical equation. In this case, the number of moles in each element is varied.
- So, in order to make the no. of moles equal , HBr present in the reactant side must be multiplied by 2.
- Hence the balanced chemical equation is :
FeS + HBr = H2S + FeBr2
HBr + FeS titration
To estimate the quantity of sulfur or iron we can perform a titration between FeS and HBr.
Apparatus used
We need a burette, conical flask, burette holder, volumetric flask, and beakers for this titration.
Titre and titrant
HBr versus FeS, HBR acts as titrant which is taken in the burette and the molecule to be analyzed is FeS which is taken in a conical flask.
Indicator
The whole titration is done in an acidic medium or acidic pH so the best suitable indicator will be phenolphthalein which gives perfect results for this titration at given pH.
Procedure
The burette was filled with standardized HBr and FeS was taken in a conical flask along with the respective indicator. HBr is added dropwise to the conical flask and the flask was shaking constantly. After a certain time when the endpoint arrived indicator changes its color and the reaction was done.
We repeat the titration several times for better results and then we estimate the iron as well as sulfur quantity by the formula V1S1 = V2S2.
HBr + FeS net ionic equation
There will be no net ionic equation for the of reaction HBr + FeS
- To derive this net ionic eq following steps are followed:
- Split the strong electrolytes into ions.
- H+ + Br– + Fe2+ + S2- = 2H+ + S2- + Fe2+ + 2Br–
- In this reaction strong electrolytes are H2S, FeBr2 , HBr and FeS .
- Cancel the spectator ions on both sides and write down the net ionic equation.
- All of the spectator ions gets cancelled so it is a case of No Reaction.
HBr + FeS conjugate pairs
The conjugate acid-base pairs of the reaction HBr+ FeS are:
- HBr (Conjugate Base) = Br–
- H2S (Conjugate Base) = HS–
HBr and FeS intermolecular forces
The intermolecular force in HBr is the strong electrostatic interaction between the proton and bromide ion, and because of the polarity, there is also some van der Waal’s attraction force. However, in the case of FeS, only covalent force is present, while in the case of H2S, only covalent force is present.
HBr + FeS reaction enthalpy
For the reaction between HBr + FeS reaction enthalpy is, -159.3 KJ/mol which can be obtained by the formula enthalpy of products – enthalpy of reactants, and here the change in enthalpy is positive.
| Molecule | Enthalpy |
| HBr | -92.3KJ/mol |
| FeBr2 | -331.8KJ/mol |
| H2S | -20.6KJ/mol |
| FeS | -100.5KJ/mol |
Is HBr + FeS a buffer solution ?
The reaction between HBr + FeS is not a buffer solution because one is strong acid but the other is not a salt of a strong or weak base, so no chance of the formation of buffer in an aqueous solution.
Is HBr + FeS a complete reaction ?
HBr + FeS is a complete reaction because the reaction results in the two final products, FeBr2 and H2S. The reaction needs some time to finish, and until all of the reactants have fully combined, no products have been formed.
Is HBr + FeS an exothermic or endothermic reaction ?
HBr + FeS is an endothermic reaction, according to the first law of thermodynamics the reaction between HBr and FeS has a positive change in enthalpy. In order to complete the reaction, the reaction takes temperature from its surroundings.
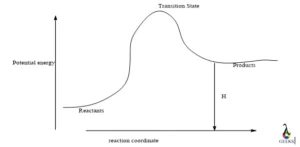
Is HBr + FeS a redox reaction ?
The reaction between HBr and FeS is a redox reaction because numerous elements are reduced and oxidized during this process, such as when bromide is transferred from reactant to product and sulfur is oxidized.
Is HBr + FeS a precipitation reaction ?
It is a precipitation reaction when HBr and FeS are combined because H2S forms at the end of the reaction. H2S is a gaseous molecule, so it cannot dissolve in water. However, at high temperatures, it precipitates at the lower part of conical shapes.
Is HBr + FeS reversible or irreversible reaction ?
The reaction between HBr and FeS is irreversible in nature. H2S gas is created in this spot as well, but it dissipated from the reaction vessel and could not interact with the ferrous chloride in a backwards manner; it could only do so in a forward direction.
Is HBr + FeS displacement reaction ?
The reaction between HBr and FeS is a single displacement reaction because sulfur was only moved from its initial position to another where Fe replaced sulfur and attached to the bromide ion to produce the desired products.
FeS + 2HBr = H2S + FeBr2
Conclusion
The HBr and FeS reaction primarily produces hydrogen sulfide and ferrous bromide, making it a crucial reaction for the production of hydrogen sulfide gas on a commercial scale. A paramagnetic solid with a yellow or brownish color, FeBr2 is an anhydrous compound.

Hello, viewers I am Mansi Sharma. I’m a medicinal chemist with a postgraduate degree. I love all facets of chemistry, and I do think that it is present everywhere, so let us explore what chemistry is all about together.
Let us connect through LinkedIn